Table 5.
Olefin CM Reactions in 2.5% aqueous PTSa
| entry | substrate | olefinic partner | product | yield (%)b |
|---|---|---|---|---|
| 1 |
|
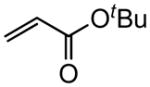
|
|
92 |
| 2 |
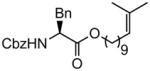
|
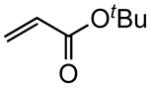
|
|
97 |
| 3 |
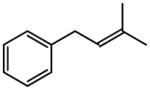
|
|
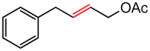
|
84 |
| 4 |
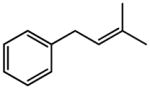
|
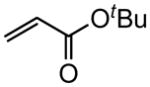
|
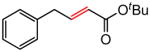
|
88 |
| 5 |
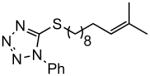
|
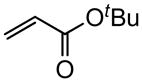
|
|
90c (55)d |
| 6 |
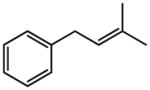
|
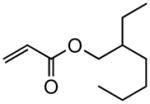
|
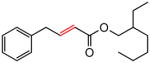
|
88 |
| 7 |
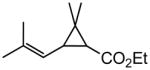
|
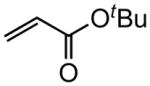
|
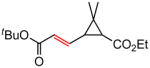
|
83c (50)d |
| 8 |
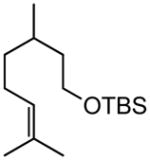
|
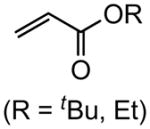
|

|
84, 86f (tBu) 82(Et) |
| 9 |
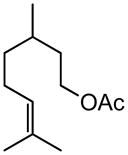
|
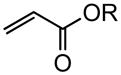
|
|
80 (tBu) 78(2-ethylhexyl) |
Reaction were conducted at 0.5 M over 12 h at 22 °C using 3 mol % Grubbs-2.
Chromatographically pure materials.
Based on recovered starting material.
Isolated.
Mixture of cis and trans isomers.
Reaction run in 3 M aqueous NaCl/PTS for 6 h.
