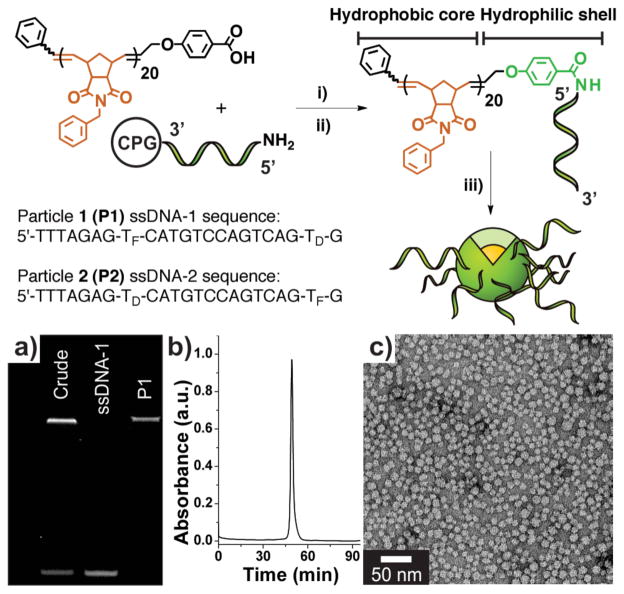
Figure 1.
Preparation of DNA-polymer amphiphiles (DPAs) and assembly of micelles. Synthesis: i) A hydrophobic polymer, terminally modified with a carboxylic acid moiety was mixed with a coupling agent and reacted with a 5′-amino modified oligonucleotide on solid support (controlled pore glass, CPG). ii) Deprotection and cleavage of the resulting DNA-polymer conjugate from solid support. iii) Dialysis of cleaved DPA into deionized water to form a mixture of micelles and free, non-conjugated nucleic acid. TF and TD correspond to fluorescein- and DABCYL-modified thymidine phosphoramidites. a) PAGE analysis. Lane 1: Crude material post-micelle (P1) formation showing conjugate (top band) and free ssD-NA (lower band). Lane 2: HPLC purified sample of ssDNA-1. Lane 3: Purified P1, isolated via size-exclusion chromatrography (SEC). b) SEC trace of purified P1 (λabs = 260 nm). c) Transmission electron micrograph of P1. See Supporting Information Figure S7 for P2 data.