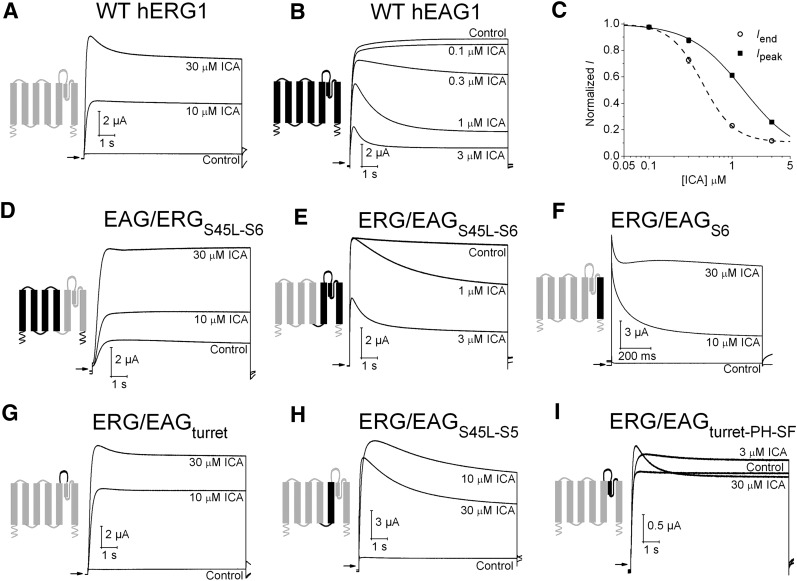
Fig. 1.
The pore domain of EAG1 and ERG1 channels determines functional response to ICA. (A) ICA activates WT hERG1 channel current. (B) ICA inhibits WT hEAG1 channel current. For traces shown in A and B, currents were activated with 10-second pulses to +30 mV from a holding potential of −100 mV. (C) [ICA]-response relationship for inhibition of hEAG1 channel currents (n = 5). ICA inhibited Iend, current at the end of a 10-second pulse to +30 mV (IC50 = 0.44 ± 0.03 μM; nH = 2.3) more potently than Ipeak, peak outward current (IC50 = 1.38 ± 0.04 μM; nH = 1.4). (D–I) Effect of ICA on EAG1/ERG1 chimera channels. In each panel, the diagram indicates regions of subunits contributed by bEAG1 (black) and hERG1 (gray). Currents were elicited with 10-second pulses to +30 mV from a holding potential of −100 mV. The fold-change in Iend induced by ICA for each chimera channel was as follows: (D) EAG/ERGS45L-S6 (1.9 ± 0.03 at 10 μM; 3.6 ± 0.6 at 30 μM, n = 3). (E) ERG/EAGS45L-S6 (0.58 ± 0.07 at 1 μM; 0.24 ± 0.04 at 3 μM, n = 4). (F) ERG/EAGS6 (9.6 ± 3.5 at 10 μM; 41 ± 11 at 30 μM, n = 4). (G) ERG/EAGturret (15 ± 1.9 at 10 μM; 26 ± 0.3 at 30 μM, n = 3). (H) ERG/EAGS45L-S5 (9.7 ± 1.6 at 10 μM; 6.1 ± 1.0 at 30 μM, n = 3). (I) ERG/EAGturret-pore helix-selectivity filter (% change in Iend: 9 ± 3 at 3 μM; 4 ± 2 at 10 μM; -3 ± 3 at 30 μM, n = 3).