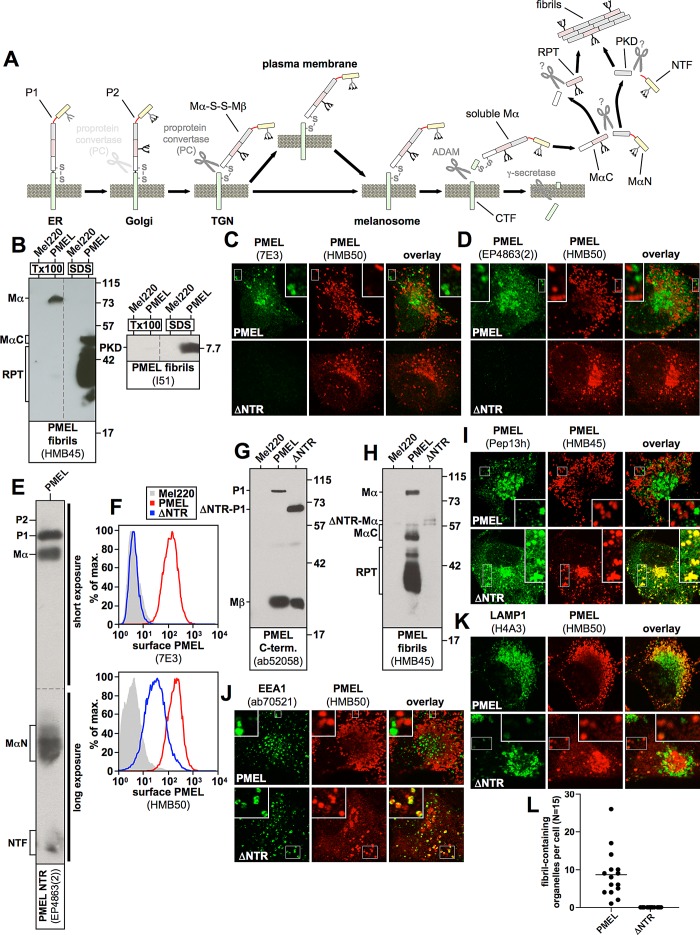
FIGURE 1:
(A) The PMEL maturation pathway. PMEL is inserted into the endoplasmic reticulum membrane in the so-called P1 form. After its release into the Golgi apparatus, N-linked oligosaccharides mature, and additional O-linked glycosylation is added, giving rise to the P2 form. Starting in the medial Golgi or in the trans-Golgi network, PMEL undergoes cleavage by a proprotein convertase, which separates the luminal fragment Mα from the membrane-standing fragment Mβ (S1 cleavage). These fragments, however, remain linked to each other by a disulfide bridge. In this form (Mα-S-S-Mβ) the protein is, either directly or via the plasma membrane, delivered into stage I melanosomes, which represent a specialized multivesicular early endosome. PMEL is originally targeted to the limiting membrane of this organelle but subsequently buds into the interior in a process dependent on the tetraspanin CD63. Next a metalloprotease of the ADAM family liberates the soluble Mα fragment from the membrane (S2 cleavage), and the remaining truncated portion of Mβ (called the CTF fragment) is degraded by γ-secretase (S3 cleavage). After this, Mα is cleaved by an unknown protease between the PKD and the RPT domain, giving rise to two halves—the N-terminal fragment, MαN, and the C-terminal fragment, MαC. Both MαN and MαC undergo further processing, which eventually liberates the PKD-containing fibrillogenic fragment, as well as a ladder of fibril-associated fragments containing the RPT domain. These two types of fragments assemble into the characteristic PMEL fibrils. The NTR is not or only to a minor extent part of the fibrils. (B) MαC, the mature RPT domain, and the mature PKD are distributed in the fibril-containing Triton X-100–insoluble fraction. Cells were extracted in 2% Triton X-100 for 1 h and centrifuged at 100,000 × g for 10 min before supernatant was removed and analyzed by SDS–PAGE and Western blotting (left lanes labeled Tx100 in both blots). The Triton X-100–insoluble pellet was resuspended in PBS/1% SDS/1% β-mercaptoethanol and incubated for 10 min at room temperature, followed by 10 min at 100°C, and analyzed on the same gel (right lanes labeled SDS in both blots). Vertical dashed lines indicate where irrelevant lanes have been removed from the image. (C, D) The indicated cell lines were analyzed by IF using antibodies against PMEL fibrils (HMB50) and either antibody 7E3, raised against full-length recombinant PMEL (C), or antibody EP4863(2), raised against a peptide located within the first 100 amino acids of the PMEL NTR (D). Note that both 7E3 and EP4863(2) do not recognize the NTR deletion mutant ΔNTR, display a staining pattern limited to the ER, Golgi, and endosomes (but not stage II melanosomes), and fail to colocalize with HMB50-reactive fibrils. (E) Western blot analysis of a lysate derived from PMEL-expressing Mel220 cells. Note that antibody EP4863(2) specifically recognizes PMEL fragments that contain the NTR, such as P1, P2, Mα, MαN, and NTF. (F) The indicated transfectants were surface labeled with antibody HMB50 against folded PMEL (bottom) or PMEL-specific antibody 7E3 (top) and analyzed by flow cytometry. Note that antibody 7E3 does not recognize construct ΔNTR. (G, H) Western blot analysis of SDS-lysed total membranes using PMEL-specific antibodies ab52058 (G) and HMB45 (H). (I–K) The indicated cell lines were analyzed by IF using antibodies against newly synthesized (Pep13h) and mature (HMB45) PMEL (I), the early endosomal marker EEA1 (ab70521) and mature PMEL (HMB50; J), or the lysosomal marker LAMP1 (H4A3) and mature PMEL (HMB50; K). Note that the HMB45/HMB50-reactive population of ΔNTR but not of wt-PMEL colocalizes with the respective newly synthesized, Pep13h-reactive population (I) partially in endosomes with intense peripheral EEA1 decoration (J; see Supplemental Table S1). Only mature wt-PMEL distributes into the characteristic melanosomal horseshoe-shaped band wrapping around the perinuclear LAMP1high zone (K). (L) Quantification of the EM analysis of Epon-embedded Mel220 transfectants showing the number of fibril-containing organelles per cell (n = 15).