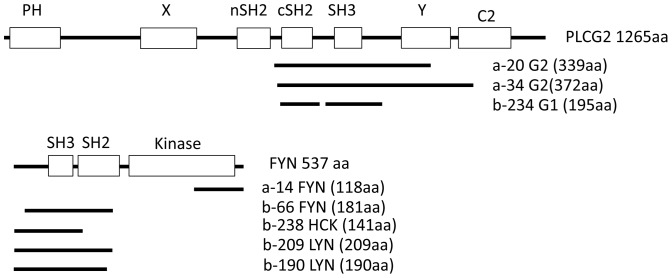
Figure 1. Clones belonging to the phosphoinositide-specific phospholipase C and the family of Src kinases isolated in two yeast two-hybrid screens.
(A) Representation of PLCg2 modular structure and the coding region of the clones identified in the Y2H. Clones named a- correspond to the first screen using as bait the full-length BANK1, clones starting with b- are the ones identified in the screen with the non autoactivating truncated protein BANK1 (331–785). The clone b-234 has a deletion of 25 aa between the cSH2 and SH3 domains. (B) Structure of the Src kinase FYN and the isolated clones belonging to this family of non- receptor kinase. PH: Pleckstrin homology domain, involved in the recruitment to membranes by binding to phosphatidylinositol containing lipids. X and Y are the two halves of the catalytic isomerase. SH2 (Src homology 2) conserved domain that typically binds to phosphorylated tyrosine residues. SH3 (Src homology 3) usually binds to proline-rich motifs. The C2 motif is present in many proteins that interact with membranes and are frequently involved in calcium dependent phospholipid binding and membrane targeting processes.