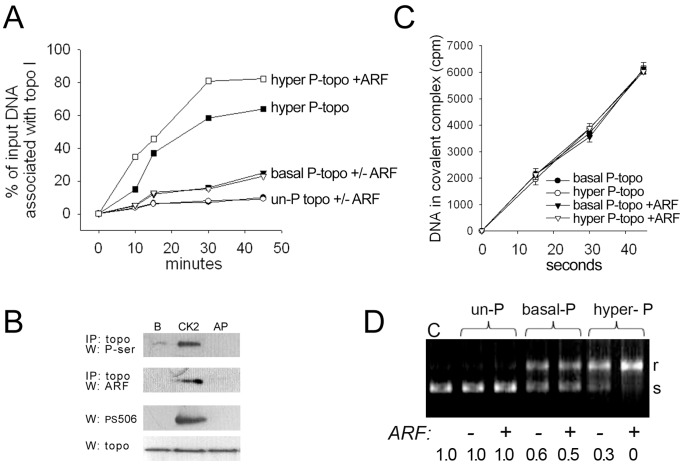
Figure 2. Effects of topo I phosphorylation status and ARF on topo I DNA association and relaxation activity.
(A) Time course of non-covalent association [low salt (75 mM NaCl) at 4°C] of various R-topo I forms (0.3 pmol) with 0.03 pmol [3H]-labeled plasmid DNA in the presence or absence of 0.3 pmol bacterially produced human ARF fusion protein: hyperphosphorylated topo I minus ARF (▪) or plus ARF (□); basal phosphorylated topo I minus ARF (▾) or plus ARF (▿); and unphosphorylated (AP-treated) topo I minus ARF (•) or plus ARF (○). (B) Row 1: Topo I immunoprecipitation (IP) with basal phosphorylated (lane “B”), hyperphosphorylated (lane “CK2”), and unphosphorylated (lane “AP”) R-topo I (1 µg) followed by phosphoserine Western; row 2: topo I IP of basal phosphorylated, hyperphosphorylated, or unphosphorylated R-topo I (1 µg) incubated with 0.14 µg ARF, followed by ARF Western; rows 3 and 4: Western analyses of PS506 and total topo I, respectively, with the same basal phosphorylated, hyperphosphorylated, and unphosphorylated R-topo I samples as in rows 1 and 2 (0.3 µg per lane) (C) Rate of topo I-catalyzed nicking of a radiolabeled suicide substrate that traps topo I and DNA in a covalent complex. Non-covalent complexes of DNA with basal or hyperphosphorylated R-topo I were preformed by incubation in low salt at 4°C in the presence or absence of recombinant ARF, then the temperature was raised to 8°C for the indicated times. Covalently linked DNA-topo I complexes were recovered by precipitation with K+SDS and quantified by scintillation counting. (D) Topo I-mediated plasmid relaxation assay performed with unphosphorylated, basal phosphorylated, and hyperphosphorylated R- topo I, followed by agarose gel electrophoresis to separate substrate and products; s = supercoiled, r = relaxed plasmid DNA.