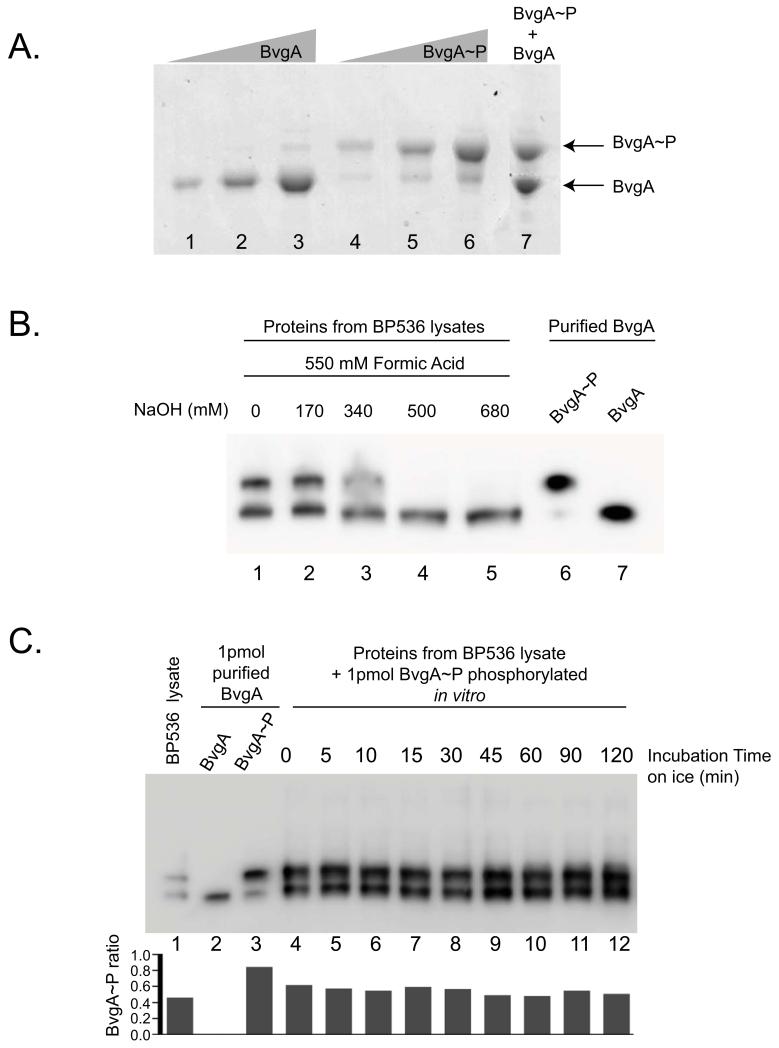
Figure 1. BvgA and BvgA~P can be separated by Phos-tag™ polyacrylamide gel electrophoresis and detected in vivo by Western analysis.
A. Phosphorylation of BvgA in vitro. A Coomassie-stained gel shows different amounts [10 pmol (lane 1 and 4), 25 pmol (lane 2 and 5), 50 pmol (lane 3 and 6)] of BvgA after treatment with buffer alone (lanes 1-3) or buffer plus 20 mM Ac~P (lanes 4-6), followed by SDS-PAGE with Phos-tag™. Lane 7 contains a mix of 40 pmol of BvgA and 40 pmol of BvgA~P.
B. BvgA phosphorylation in B. pertussis. Cells were lysed and processed as described in Experimental Procedures using different amounts of NaOH (lanes 1-5) before electrophoresis on a Phos-tag™ gel and Western analysis. As a control, 1 pmol of purified BvgA~P (lane 6) and BvgA (lane 7) was loaded on the gel.
C. Stability of BvgA~P during cell lysate processing. One frozen aliquot of B. pertussis BP356 cells (harvested from 1 mL culture at OD600 0.15) was mixed with 15 pmol of in vitro phosphorylated BvgA. This combination of purified BvgA~P and B. pertussis cells was treated with 550 mM formic acid and 170 mM NaOH in a total volume of 60 μl for lysis (as described in Experimental Procedures) and incubated on ice for the indicated amount of time. From this mixture, 4 μl (containing 1 pmol of purified BvgA~P plus the in vivo B. pertussis BvgA~P) were electrophoresed on the Phos-tag™ gel. BvgA was detected by Western analysis. Lane 1 shows the pellet lysate with no purified BvgA~P added, to indicate the starting ratio of BvgA~P to BvgA. Lanes 2 and 3 correspond to 1 pmol of purified BvgA or BvgA~P, respectively. The graph represents the ratio of BvgA~P on the total amount of BvgA protein for each lane.