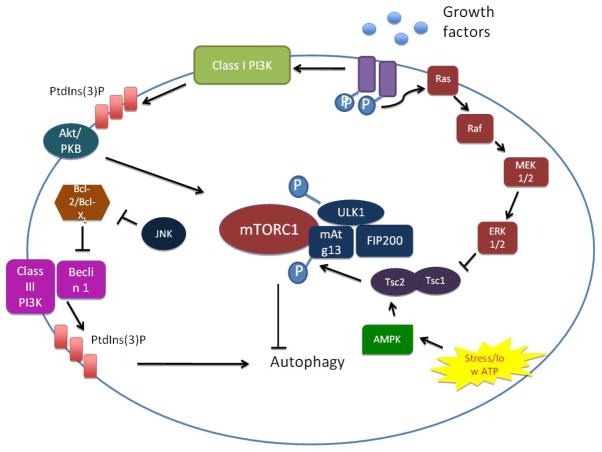
Figure 2.
mTORC-dependent regulation of autophagy
Autophagy is induced under stress conditions such as nutrient or ATP depletion. The rapamycin-sensitive serine/threonine protein kinase mTORC1 mediates the nutrient-sensitive regulation of autophagy. In unstressed conditions, or in the presence of ample nutrients, autophagy levels are maintained at a low level through activation of mTORC1, which inactivates the ULK1/2-Atg13-FIP200 complex. Nutrient starvation, on the other hand, instigates dissociation of mTORC1 and partial dephosphorylation of ULK1/2. Activated ULK1/2 phosphorylates the Atg13-FIP200 complex, which instigates conformational changes essential for autophagy. The PI3K pathway mediates upstream regulation of the mTOR pathway. Growth factor binding to receptor tyrosine kinases instigates their autophosphorylation and activation, which leads to the activation of the class I PI3K and the generation of phosphatidyinositol-3-phosphate (PI3P). These changes provoke the recruitment of Akt, which activates mTORC1 and suppresses autophagy. However, the class III PI3K (Vps34) stimulates autophagy independently of mTORC1, through the generation of PI3P. Certain members of the MAPK pathway, including ERK1/2 and JNK, as well as AMPK, also modulate autophagy through mTORC1.