Abstract
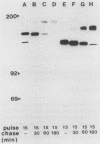
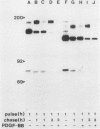
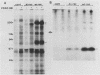
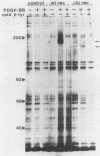
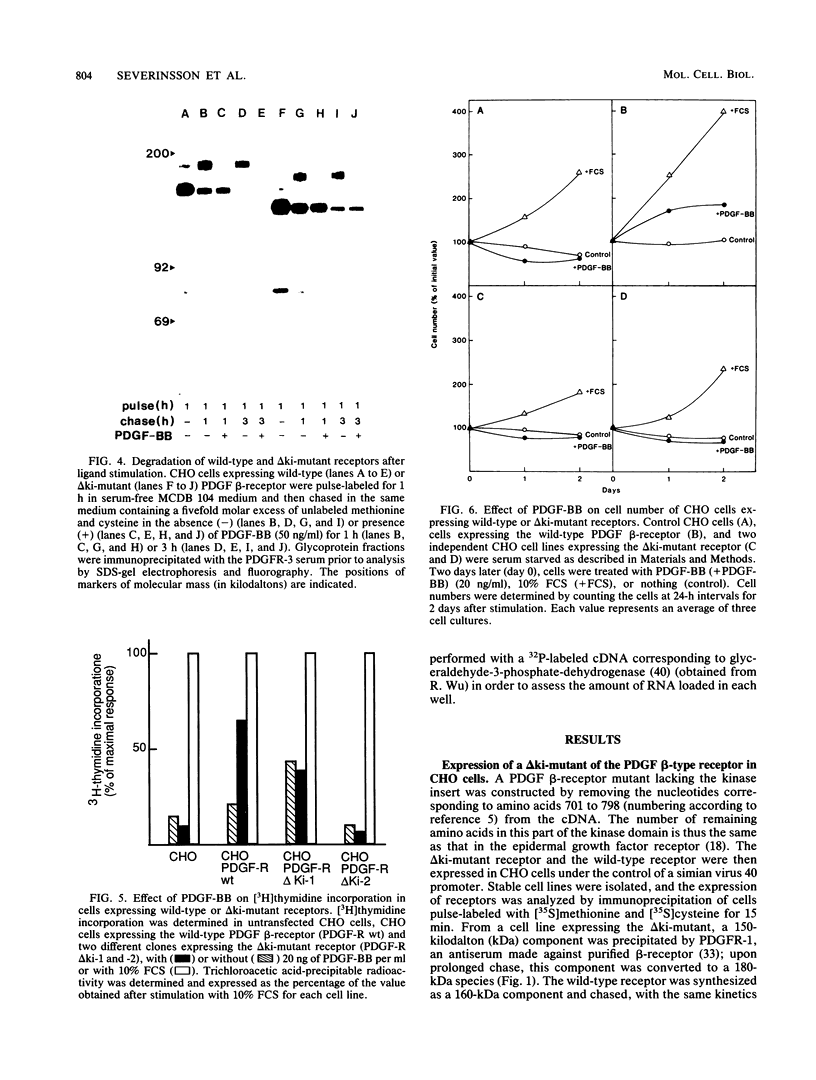
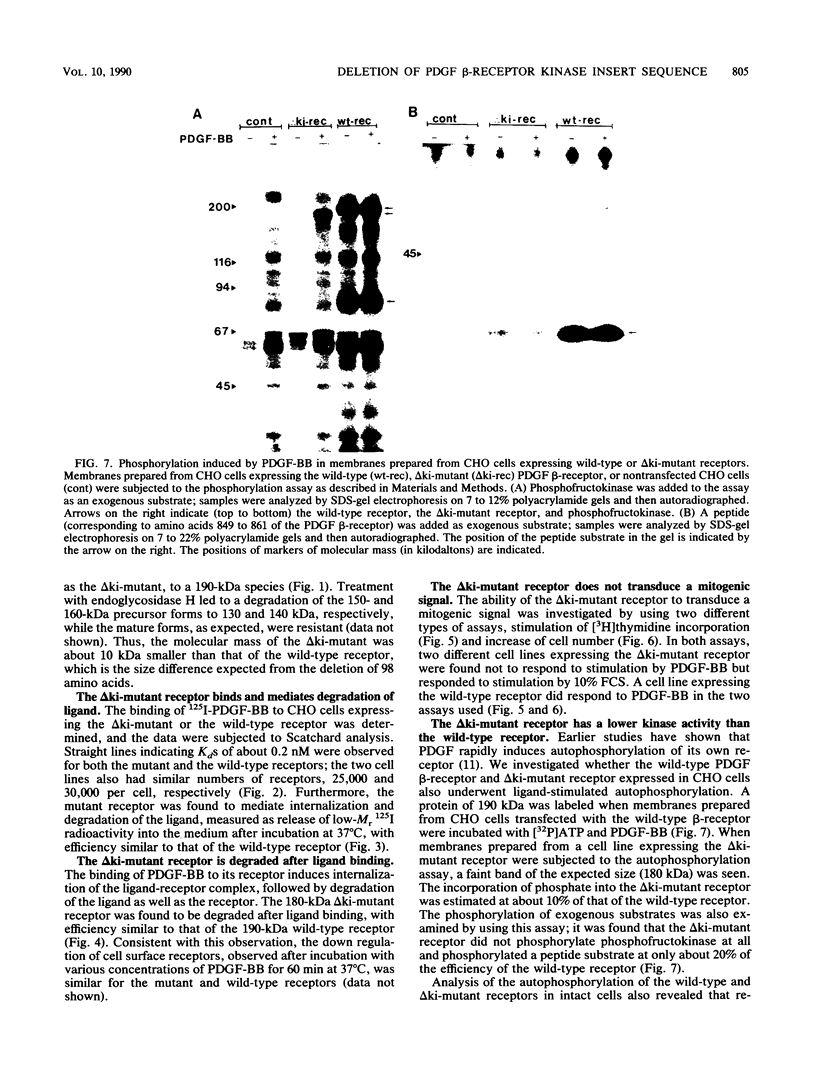
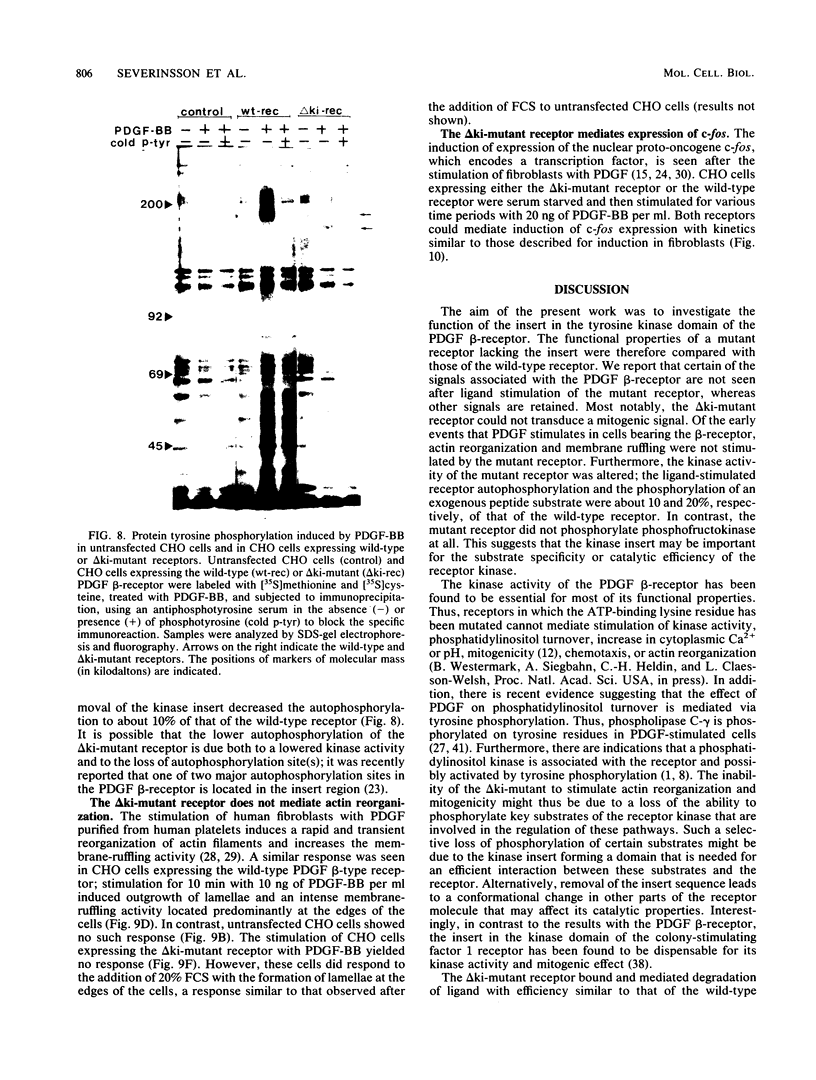
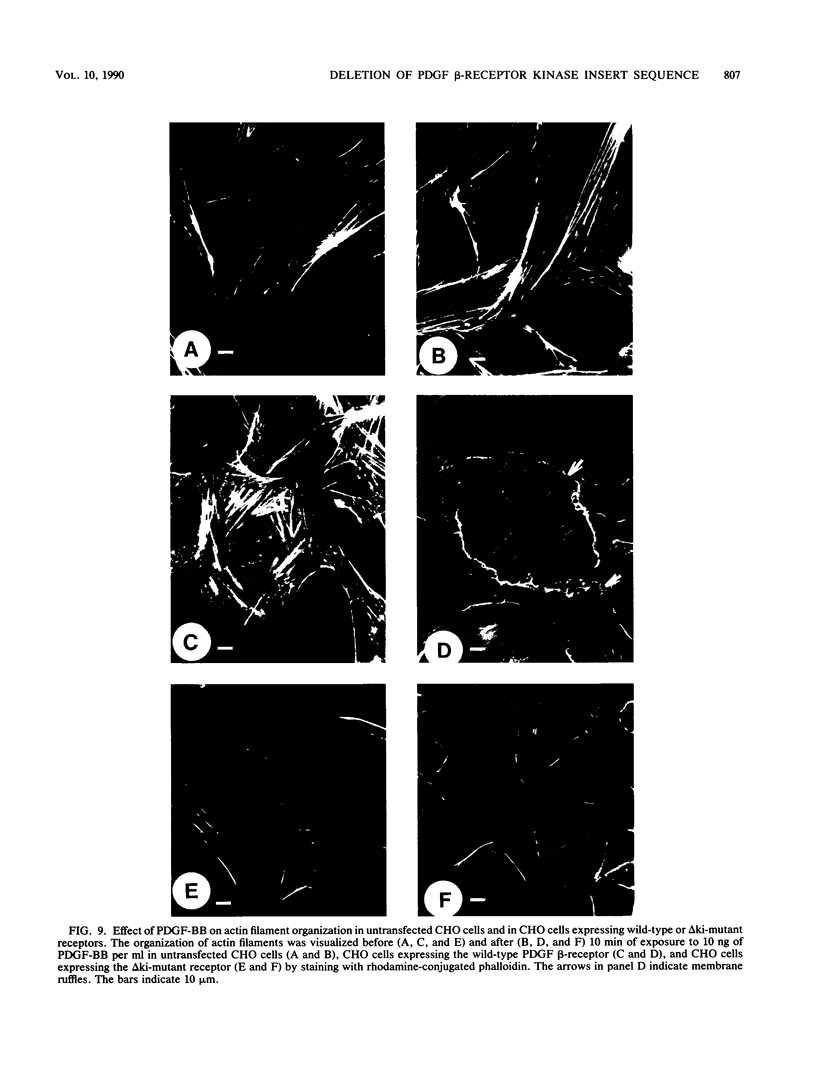
A characteristic feature of the platelet-derived growth factor (PDGF) beta-receptor is the presence of an insert sequence in the protein tyrosine kinase domain. A receptor mutant which lacks the entire insert of 98 amino acids was expressed in CHO cells, and its functional characteristics were compared with those of the wild-type receptor. The mutant receptor bound PDGF-BB with high affinity and mediated internalization and degradation of the ligand with efficiency similar to that of the wild-type receptor but did not transduce a mitogenic signal. It was found to display a decreased autophosphorylation after ligand stimulation and had a decreased ability to phosphorylate exogenous substrates; phosphofructokinase was not phosphorylated at all, whereas a peptide substrate was phosphorylated, albeit at a lower rate compared with phosphorylation by the wild-type receptor. Furthermore, the mutant receptor did not mediate actin reorganization but mediated an increase in c-fos expression. The data indicate that the insert in the kinase domain of the PDGF beta-receptor is important for the substrate specificity or catalytic efficiency of the kinase; the deletion of the insert interferes with the transduction of some, but not all, of the signals that arise after activation of the receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Morén A., Severinsson L., Ek B., Ostman A., Betsholtz C., Heldin C. H. cDNA cloning and expression of a human platelet-derived growth factor (PDGF) receptor specific for B-chain-containing PDGF molecules. Mol Cell Biol. 1988 Aug;8(8):3476–3486. doi: 10.1128/mcb.8.8.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Hammacher A., Westermark B., Heldin C. H., Nistér M. Identification and structural analysis of the A type receptor for platelet-derived growth factor. Similarities with the B type receptor. J Biol Chem. 1989 Jan 25;264(3):1742–1747. [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Use of an antiserum against phosphotyrosine for the identification of phosphorylated components in human fibroblasts stimulated by platelet-derived growth factor. J Biol Chem. 1984 Sep 10;259(17):11145–11152. [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Barr P. J., Williams L. T. Role of tyrosine kinase and membrane-spanning domains in signal transduction by the platelet-derived growth factor receptor. Mol Cell Biol. 1988 Dec;8(12):5126–5131. doi: 10.1128/mcb.8.12.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Keating M. T., Ives H. E., Williams L. T. Platelet-derived growth factor receptors expressed by cDNA transfection couple to a diverse group of cellular responses associated with cell proliferation. J Biol Chem. 1988 Jan 25;263(3):1482–1487. [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gronwald R. G., Grant F. J., Haldeman B. A., Hart C. E., O'Hara P. J., Hagen F. S., Ross R., Bowen-Pope D. F., Murray M. J. Cloning and expression of a cDNA coding for the human platelet-derived growth factor receptor: evidence for more than one receptor class. Proc Natl Acad Sci U S A. 1988 May;85(10):3435–3439. doi: 10.1073/pnas.85.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: three isoforms and two receptor types. Trends Genet. 1989 Apr;5(4):108–111. doi: 10.1016/0168-9525(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A., Hammond S. L., Ham R. G. Improved medium for clonal growth of human diploid fibroblasts at low concentrations of serum protein. In Vitro. 1977 Jul;13(7):399–416. doi: 10.1007/BF02615100. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mellstroöm K., Höglund A. S., Nistér M., Heldin C. H., Westermark B., Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983 Oct;4(5):589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- Mellström K., Heldin C. H., Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988 Aug;177(2):347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Ostman A., Bäckström G., Fong N., Betsholtz C., Wernstedt C., Hellman U., Westermark B., Valenzuela P., Heldin C. H. Expression of three recombinant homodimeric isoforms of PDGF in Saccharomyces cerevisiae: evidence for difference in receptor binding and functional activities. Growth Factors. 1989;1(3):271–281. doi: 10.3109/08977198908998003. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L., Beckmann M. P., Faulders B., Ostman A., Ek B., Heldin C. H. Purification of the receptor for platelet-derived growth factor from porcine uterus. J Biol Chem. 1987 Mar 5;262(7):2929–2932. [PubMed] [Google Scholar]
- Severinsson L., Claesson-Welsh L., Heldin C. H. A B-type PDGF receptor lacking most of the intracellular domain escapes degradation after ligand binding. Eur J Biochem. 1989 Jul 1;182(3):679–686. doi: 10.1111/j.1432-1033.1989.tb14879.x. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Reedijk M., Rothwell V., Rohrschneider L., Pawson T. The unique insert of cellular and viral fms protein tyrosine kinase domains is dispensable for enzymatic and transforming activities. EMBO J. 1989 Jul;8(7):2029–2037. doi: 10.1002/j.1460-2075.1989.tb03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett M. A., Blacher R., Burke R. L., Caput D., Chu C., Dina D., Hartog K., Kuo C. H., Masiarz F. R., Merryweather J. P. Characterization of the polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of the human kidney cDNA. DNA. 1985 Oct;4(5):333–349. doi: 10.1089/dna.1985.4.333. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]