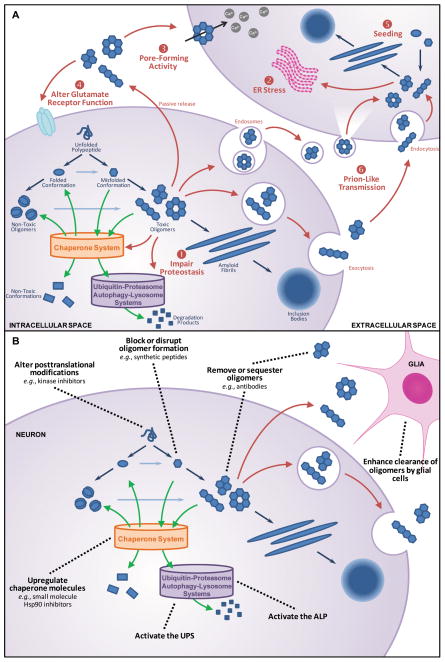
Figure 1. α-Syn oligomers and Parkinson disease.
(A) Formation and toxicity of α-syn oligomers. Protein aggregation in neurodegenerative diseases is initiated by aberrant protein folding which leads to the formation of oligomers and eventually amyloid fibrils and inclusions bodies (blue arrows). Certain oligomeric species are toxic to cells by mechanisms that include (1) impairment of proteostasis, (2) chronic endoplasmic reticulum (ER) stress, (3) pore formation, (4) glutamate receptor dysfunction, (5) seeding with (6) prion-like transmission (red arrows), all of which may combine in the pathogenic process of cell death and transmission. Endogenous cellular systems which can reduce oligomer levels are the chaperone network, the ubiquitin-proteasomal system (UPS), and the autophagy-lysosomal pathway (ALP) (green arrows).
(B) Potential treatment strategies which reduce toxic α-syn oligomers to slow or prevent neurodegeneration. These strategies target different steps along the protein aggregation pathway, as well as intracellular and/or extracellular pools of α-syn oligomers. The primary goal of treatment is to reduce toxic oligomer levels directly or indirectly by preventing oligomer formation, disrupting already formed oligomers, promoting degradation of toxic oligomers or conversion of toxic oligomers to non-toxic oligomers, and sequestering or clearing oligomers by antibody or cell-based mechanisms to prevent cell-to-cell transmission (black dotted lines).