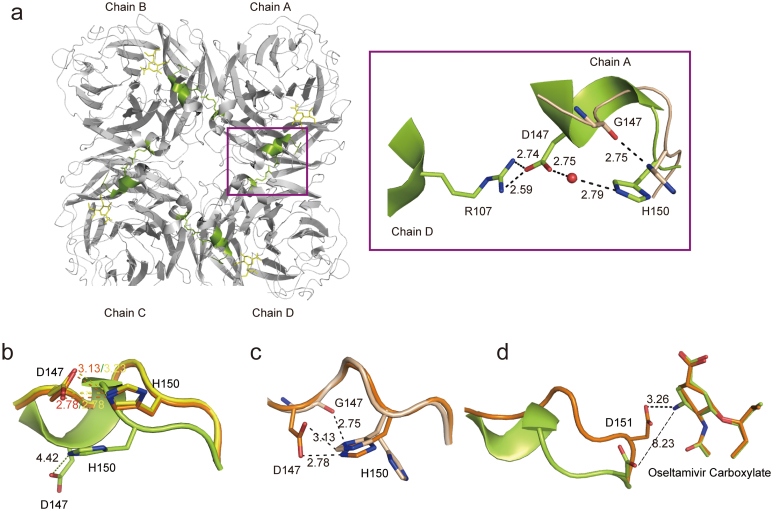
Figure 2. Structural basis of an open 150-loop in N2 neuraminidase.
(a) Overview of the open form of 150-loop in the N2-oseltamivr complex structure with an emphasis on the intermolecular interaction indicated with a violetpurple box. The residues of N2-wild type are shown in limon, while the residues of N2-G147 are shown in wheat. The water molecule is shown in red sphere. (b) Superposition of the 150-loop conformation between free N2 (yellow), N2-oseltamivir closed form (orange) and N2-oseltamivir open form (limon). Compare the interaction between D147 and H150. (c) Superposition of 150-loop conformation between N2-oseltamivir closed form (orange) and N2-G147 after a 1 hour soak with 20 mM oseltamivir carboxylate (wheat). Compare the interaction between Residue 147 and 150. (d) Superposition of 150-loop conformation between N2-oseltamivir closed form (orange) and N2-oseltamivir open form (limon). Compare the interaction between D151 and amino group of oseltamivir carboxylate. The side chain of the residues is displayed in stick representation with hydrogen bonds and salt bridges indicated by dotted lines. The interaction with a distance greater than 3.5 Å is shown as a broken line. The shared residues are highlighted in black and labeled.