Table 4.
Synthesis of Secondary Aromatic Aminesa
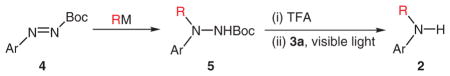
| ||||
|---|---|---|---|---|
| Entry | 4 | RM | 5 (Yield %) | 2 (Yield %) |
| 1 |
 4n |
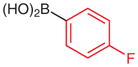
|
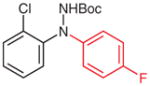 5n (95) |
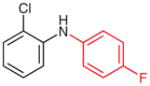 2n (91)b |
| 2 |
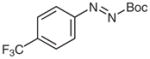 4o |
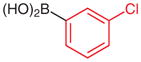
|
 5o (95) |
 2o (87)b |
| 3 |
 4p |
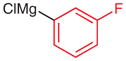
|
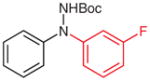 5p (96) |
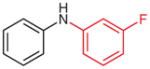 2p (84)b |
| 4 |
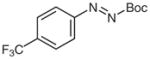 4q |
PhMgBr |
 5q (96) |
 2q (85)b |
| 5 |
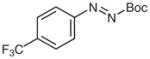 4r |
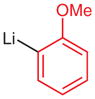
|
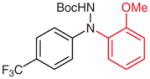 5r (79) |
 2r (65)b |
| 6 |
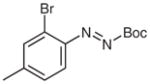 4s |
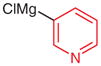
|
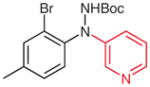 5s (73) |
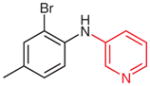 2s (55) |
| 7 |
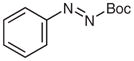 4t |
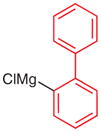
|
 5t (50) |
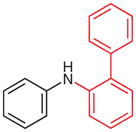 2t (72)b |
| 8 |
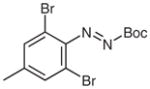 4u |
BuMgCl |
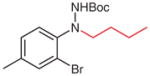 5u (92) |
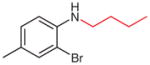 2u (70)b |
| 9 |
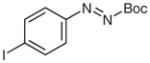 4v |
BuMgCl |
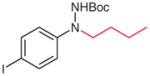 5v (95) |
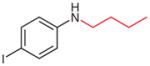 2v (21)b,c |
| 10 |
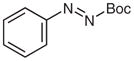 4w |
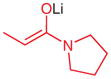
|
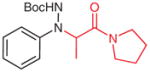 5w (71) |
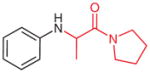 2w (51)b |
Reactions were performed on 0.3–0.5 mmol scale in 3–5 mL of MeCN–MeOH (1:1 v/v) with 2 mol% 3a. A household 13 W light bulb was used as the light source. Reaction time ranged from 15 to 48 h.
Due to the instability of these hydrazine derivatives, the crude products were subjected to the cleavage reaction directly after Boc-deprotection. The yields refer to overall yields for the two steps.
Another 2 mol% of 3a was added after 10 h; 43% of hydrazine was recovered.
