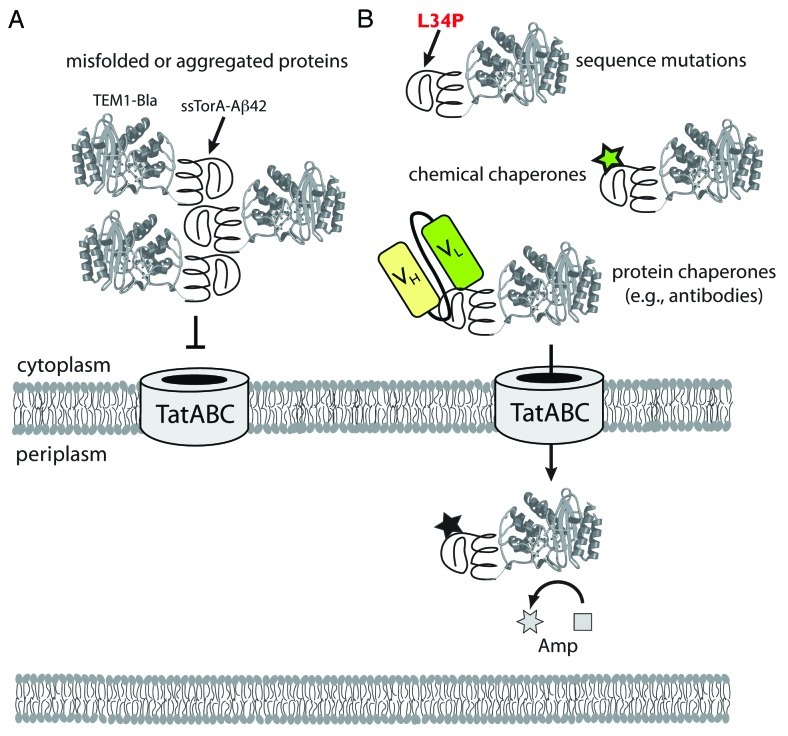
Figure 1. Use of the Tat folding sensor for uncovering structural probes of protein misfolding and aggregation. (A) Chimeras comprised of aggregation-prone test proteins (e.g., ssTorA-Aβ42-Bla) expressed in the cytoplasm readily aggregate via self-assembly of the test proteins. This aggregation is “sensed” by the Tat translocase, and export of these aggregated substrates is blocked. (B) In the presence of stabilizing mutations or exogenous factors such as chemical or protein chaperones that bind to the test protein and prevent aggregation, chimeras such as ssTorA-Aβ42-Bla remain soluble and are efficiently exported to the periplasm where Bla hydrolyzes Amp. The extent to which protein chimeras are exported, and thus the amount of Bla that is transferred to the periplasm, determines the resistance phenotype of the host cells. In this fashion, in vivo stability of proteins is directly linked to antibiotic resistance. The approach facilitates the identification of mutations and other factors (e.g., chemical probes, antibodies) that stabilize test proteins, all without any prior structural or functional knowledge of the test protein.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.