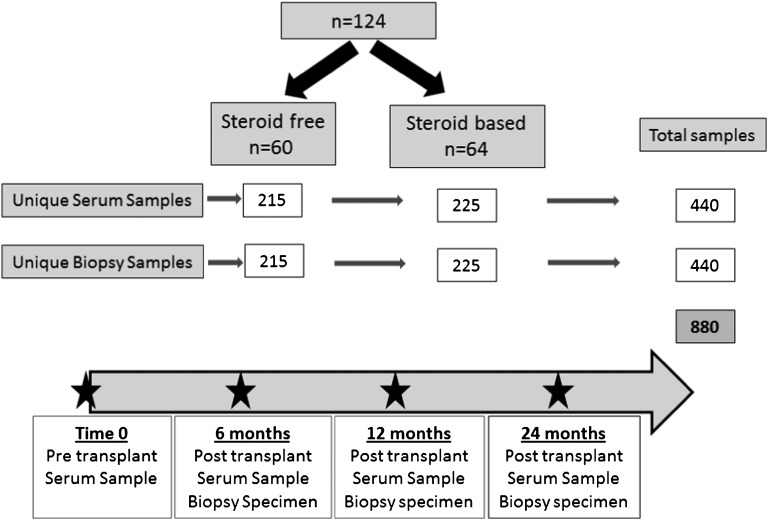
Figure 1.
Study outline. This study used 440 serum samples and 440 matched blinded biopsy scores for CADI, CNIT, Banff rejection grading, and C4d stains on 440 matched protocol biopsies from the SNSO1 multicenter randomized trial of SF and SB immunosuppression in pediatric renal transplantation.15,16 Samples and biopsies were assayed at 0 (pretransplant), 6, 12, and 24 months post-transplantation. Of 130 patients in the trial, 124 patients had at least three of four sera samples collected and were included in the analysis.