Abstract
Objective
To compare the applicability of the SYBR Green-I assay with the standard schizont maturation assay, for determination of sensitivity of Plasmodium vivax (P. vivax) to chloroquine and a new antifolate WR 99210.
Methods
The study was conducted at Mae Tao Clinic for migrant workers, Tak Province during April 2009 to July 2010. A total of 64 blood samples (1 mL blood collected into sodium heparinized plastic tube) were collected from patients with mono-infection with P. vivax malaria prior to treatment with standard regimen of a 3-day chloroquine. In vitro sensitivity of P. vivax isolates was evaluated by schizont maturation inhibition and SYBR Green-I assays.
Results
A total of 30 out of 64 blood samples collected from patients with P. vivax malaria were successfully analyzed using both the microscopic schizont maturation inhibition and SYBR Green-I assays. The failure rates of the schizont maturation inhibition assay (50%) and the SYBR Green-I assay (54%) were similar (P=0.51). The median IC10s, IC50s and IC90s of both chloroquine and WR99210 were not significantly different from the clinical isolates of P. vivax tested. Based on the cut-off of 100 nM, the prevalences of chloroquine resistance determined by schizont maturation inhibition and SYBR Green-I assays were 19 and 11 isolates, respectively. The strength of agreement between the two methods was very poor for both chloroquine and WR99210.
Conclusions
On the basis of this condition and its superior sensitivity, the microscopic method appears better than the SYBR Green-I Green assay for assessing in vitro sensitivity of fresh P. vivax isolates to antimalarial drugs.
Keywords: Plasmodium vivax, Chloroquine, In vitro sensitivity, Schizont maturation inhibition assay, SYBR Green-I assay, Fresh isolate, Antifolate, Malaria, Antimalarial drug, Standard regimen
1. Introduction
Plamodium vivax (P. vivax) is responsible for approximately 70 to 80 million cases of malaria worldwide annually, and is the major cause of human malaria in parts of Pacific region and Central and South America[1],[2]. The blood schizontocide chloroquine and tissue schizontocide primaquine have remained the mainstay chemotherapeutics for treatment of P. vivax infection in Thailand for more than 60 years with reserved clinical efficacy of virtually 100%[3]. Nevertheless, accumulating reports of chloroquine resistance to P. vivax in other parts of the world during the past three decades[4] emphasize the need for closely and continuously monitoring of therapeutic efficacy of chloroquine. Although in vivo drug efficacy study is considered the gold standard for assessment of drug resistance in malaria parasites, it is costly and time-consuming. In vitro drug sensitivity test provides a valuable adjunct to in vivo drug susceptibility test, especially when comparing temporal and spatial variations in Plasmodium spp. susceptibility to antimalarials. Therefore, there is a need to develop a quick and cost-effective alternative mean for monitoring of drug resistance, and in addition, screening system for new antimalarial candidates should also be performed. Major advantage includes its ability to assess the intrinsic parasites' responses to drugs without interference from host factors such as immunity and pharmacokinetics.
Simple in vitro susceptibility tests for Plasmodium falciparum (P. falciparum) have been available since 1968[5]. However, the development of similar in vitro susceptibility tests for P. vivax has been problematic due to the poor in vitro growth of P. vivax. Recent advances in in vitro culture techniques (i.e., specially modified and serum-enriched media) have enabled the short-term culture of P. vivax [6]. Through the removal of leukocytes and enrichment of the growth media, it is now possible to culture P. vivax from ring stage to schizont stage. This has enabled the successful development of the two in vitro test systems for assessing P. vivax sensitivity to antimalarials by Tasanor and Russell et al[7],[8]. Either assay method relies on the microscopic examination, but with different assay end points (schizont maturation and growth inhibition, respectively). The schizont maturation inhibition assay has been used as a standard for assessing sensitivity of P. vivax to antimalarial drugs but it poses a constraint with regard to the absence of P. vivax intravascular sequestration of the higher stages of intraerythrocytic schizogony. The test system previously reported by our group based on growth inhibition[7] reflects the age composition of the parasite population and its progression over the duration of the drug response test. Both methods however, suffer from the requirement of highly experienced and skilled microscopists and extensively labour-intensive work. Recently, the less labour-intensive fluorescent-based assay methods[9],[10] have been successfully applied for sensitivity assessment of P. falciaprum to antimalarial drugs. The aim of the study was to compare the applicability of the fluorescent-based SYBR Green-I assay with the standard schizont maturation assay, for assessment of sensitivity of P. vivax to chloroquine and a new antifolate WR 99210.
2. Materials and methods
2.1. Sample collection
The study was conducted at Mae Tao Clinic for migrant workers, Tak Province during April 2009 to July 2010. The study was approved by the Ethics Committee of Ministry of Public Health of Thailand. A total of 64 blood samples (1 mL blood collected into sodium heparinized plastic tube) were collected from patients with mono-infection with P. vivax malaria prior to treatment with standard regimen of a 3-day chloroquine. Inclusion criteria included a parasitaemia of 1 000-100 000 parasites/µL blood, no signs of severe disease, and no antimalarial treatment during the preceding four weeks. Written informed consent for study participation was obtained from all patients. Blood films were stained with Giemsa stain and examined by light microscope. Asexual stages of P. vivax were counted against 1 000 erythrocytes in thin blood films or against 200 white blood cells in thick films.
2.2. In vitro drug sensitivity assay
The schizont maturation inhibition and SYBR Green-I assays were performed on all P. vivax field isolates collected from all patients using a modified method of Russell and Benett et al[8],[11]. P. vivax field isolates were tested for their sensitivities against chloroquine and WR99210. Drug plates were prepared fresh to avoid possible degradation. A stock solution of each drug was prepared in 1% dimethyl sulfoxide (DMSO) and was subsequently diluted in RPMI 1640 medium to obtain the desired drug concentrations. Fifty microliters of the final drug solution were added to each well of a 96-well microtiter plate. This plate contained varying concentrations of drug in each column and well A was free of drug and served as control. Wells B-H contained ascending concentrations of drug, each concentration of which was tested in triplicate. The concentration ranges for each drug used were 0-10 000 nM for chloroquine (chloroquine phosphate: Liverpool School of Tropical Medicine, University of Liverpool, UK) and 0-2 560 nM for WR99210 (Jacobus Pharmaceutical Inc, Princeton, NJ, USA).
2.2.1. Schizont maturation inhibition assay
A 1 mL blood sample was mixed with phosphate-buffered saline at the ratio of 1:1 and added to the CF11 column (a 10-mL syringe tipped with glass wool and filled with CF11 cellulose powder (Whatman, Florham Park, NJ, USA). The supernatant was then removed and the pellet was resuspended in RPMI 1640 medium (Gibco, USA). The blood mixture was centrifuged and the supernatant was removed. The pellet was then resuspended in human AB serum to obtain a haematocrit of 40%. The blood-serum suspension was mixed with McCoy's 5A medium (Gibco, USA) at the ratio of 1:10. The concentrations of folic acid and p-aminobenzoic acid in McCoy's medium were 10 and 1 mg/L, respectively. Fifty microliters of this mixture were added to each well of a 96-well microtiter plates pre-dosed with drug. The tested plate was incubated at 37.5 °C in a candle jar containing 5% CO2 for 24-36 h depending on the stage of the parasite before culturing. After incubation, a thick blood film was prepared from each well and the number of normal schizonts (containing > 8 nuclei) per 200 asexual stage parasites was counted. The number of schizonts in each well that contained drug was compared with that in the control well and expressed as a percentage of the control.
2.2.2. SYBR Green-I assay
Plasma and buffy coat were separated from blood samples by centrifugation and packed red cells were washed twice with RPMI 1640. The pellet was resuspended in human AB serum to obtain 40% haematocrit and was then diluted to 1% in McCoy's 5A medium containing 30% AB serum. Fifty microliters of this mixture were added to each well of pre-dosed drug plates and the tested plate was incubated at 37 °C in a candle jar with 5% CO2 for 24-36 h. After incubation, the tested plate was frozen at -20 °C until analysis. Prior to assay, 100 µL of the fluorescent haemolysis reagent (0.01% of fluorescent dye SYBR green-I (Sigma, U.S.A.) diluted in hemolysis reagent containing 20 mM Tris pH 7.5, 5 mM EDTA, 0.008% saponin and 0.08% tritonX-100) was added to each well. Fluorescent intensity was dertermined at the excitation and emission wave lengths of 485 and 530 nm, respectively. Mean values were calculated from duplicate results of fluorescent intensity of each drug and subtracted from mean of positive control (200 nM artesunate). The mean of fluorescent intensity of each drug concentration was compared with negative control and calculated to % growth inhibition.
The dose-response curves obtained from both assays were analyzed by nonlinear regression analysis using CalcuSynTM software (BiosoftTM, Cambridge, UK). The log-transformed concentration and probit-transformed inhibition data were processed as linear regressions. The results were expressed as inhibitory concentrations (IC) 10, 50 and 90 which are defined as the concentrations of chloroquine or WR99210 producing 10%, 50% and 90% inhibition of parasite development as compared with the control.
2.3. Statistical analysis
For all the clinical isolates, the interpretable results were obtained from both the schizont maturation inhibition and SYBR Green-I assays. The geometric means of the IC10s, IC50s and IC90s of each drug tested were compared by Mann-Whitney U tests. The strength of agreement between IC10s, IC50s and IC90s values for both assays was determined using Pearson's correlation test. The statistical significance level was set at P=0.05 for all tests.
3. Results
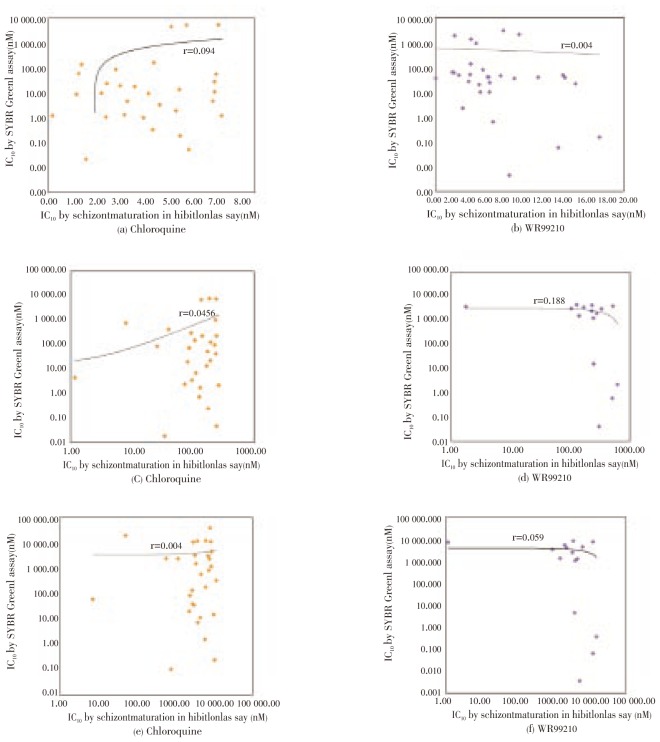
A total of 30 out of 64 blood samples collected from patients with P. vivax malaria were successfully analysed by both the microscopic schizont maturation inhibition and SYBR Green-I assays. The failure rates of the schizont maturation inhibition assay (50%) and the SYBR Green-I assays (54%) were similar (P=0.51). Comparisons of the IC10s, IC50s and IC90s for these isolates obtained by the two methods were illustrated in Table 1. The median IC10s, IC50s and IC90s of both chloroquine and WR99210 were not significantly different for clinical isolates of P. vivax tested. Based on the cut-off IC50 of 100 nM, the prevalences of chloroquine resistance determined by schizont maturation inhibition and SYBR Green assays were 19 and 11 isolates, respectively. SYBR Green-I assay was able to detect 8 out of 11 chloroquine-sensitive isolates identified by schizont maturation inhibition assay. The strength of agreement between the two methods was very weak for both chloroquine and WR99210. For chloroquine, Pearson's correlation coefficients (r) for IC10s, IC50s and IC90s were 0.094, 0.046, and 0.004, respectively. The corresponding values for WR99210 were 0.004, 0.188 and 0.059 nM, respectively (Figure 1).
Table 1. IC10, IC50 and IC90 values (nM) of chloroquine and WR99210 in P. vivax isolates by schizont maturation inhibition and SYBR Green-I assays.
| IC (nM) | Parameters | Chloroquine |
WR 99210 |
||
| Schizont maturation inhibition assay | SYBR Green-I assay | Schizont maturation inhibition sssay | SYBR Green-I assay | ||
| IC10 | Geometric mean | 3.40 | 3.59 | 4.89 | 4.97 |
| Median (95% CI) | 4.22 (0.19-7.14) | 3.50 (0.00-4 803.00) | 5.75 (0.04-17.82) | 7.88 (0.00-2.06) | |
| IC50 | Geometric mean | 99.87 | 33.09 | 122.00 | 50.65 |
| Median (95% CI) | 134.71 (1.17-264.99) | 63.16 (0.02-7.55) | 139.95 (0.21-523.00) | 181.21 (0.00-2 650.00) | |
| IC90 | Geometric mean | 2 929.46 | 305.18 | 2 566.90 | 514.74 |
| Median (95% CI) | 4 134.00 (7.16-11 535.00) | 703.00 (0.10-38 737.00) | 3 406.00 (1.17-15 356.00) | 2 765.00 (0.00-37 680) | |
Figure 1. Scatter plots of the correlation between IC10s (a, b), IC50s (c, d) and IC90s (e, f) of chloroquine and WR99210 in 30 P. vivax isolates by schizont maturation inhibition and SYBR Green-I assays.
4. Discussion
The success rate of in vitro sensitivity tests observed in the current study was relatively low (46%-50%). The low success rate is possibly due to variation in asynchronicity of parasite isolates in this area[12]. The in vitro sensitivity data based on schizont maturation inhibition assay demonstrated more or less the stability of sensitivity of P. vivax isolates in this area of Thailand to chloroquine since the year 2002[13]. It was noted that the IC50 of chloroquine in P. vivax was about 2-4 fold of that of P. falciparum, but the variation is probably higher with P. vivax. This may imply intrinsic characteristic (innate resistance) of P. vivax in response to chloroquine. The in vitro cut-off value defining clinically relevant chloroquine resistance in P. vivax malaria has yet to be clearly defined. For P. falciparum, cut-off IC50 of 100 nM was used to define chloroquine resistance. Based on this criteria, 19 out of 30 isolates (63.3%) determined by schizont maturation inhibition assay showed IC50 of greater than 100 nM.
WR99210 is a novel inhibitor of enzyme dihydrofolate reductase (DHFR) in malaria parasites. In vitro sensitivity of this compound was assessed in our study in view of previous reports showing its promise as a possible treatment of P. vivax malaria. The drug shows activity against the most pyrimethamine-resistant P. falciparum strains and is the extremely effective inhibitor of the P. vivax DHFR including mutations that confer high-level resistance to pyrimethamine[14]. Median (95% CI) IC50 of WR99210 in P. vivax isolates collected in the present study was similar to our previous observation in the same area[15]. The relatively poor in vitro susceptibility of P. vivax to WR99210 could be explained by the slow action of this drug and/or the innate resistance as well as the presence of p-aminobenzoic acid and folate in the media used which acted as competitive antagonists of antifolate activity[16]. The observed in vitro IC50 values of WR99210 therefore, may not reflect the actual in vivo sensitivity of P. vivax as the medium used was supplemented with folic acid.
In the present study, the correlation of the two in vitro sensitivity assays based on SYBR Green-I and schizont maturation inhibition of P. vivax fresh isolates in Thailand to chloroquine and WR99210 was investigated. Practically, the SYBR Green-I assay would overcome many of the disadvantages to microscopic assays and might be an alternative method for assessing sensitivity of P. vivax to antimalarial drugs, especially in malaria-endemic countries. Our study provides, for the first time, an assessment of the SYBR Green-I based fluorescence assay under routine condition in fresh P. vivax isolates. Unfortunately, the assay could detect only 8 out of 19 (42.1%) chloroquine-resistant isolates identified by schizont maturation inhibition assay. In addition, marked variation in IC10s, IC50s, and IC90s was observed when compared with results obtained from schizont maturation inhibition assay. Markedly poor correlation between IC10s, IC50s and IC90s obtained from SYBR Green-I and schizont maturation inhibition assays was found. The poor performance of the SYBR Green-I method is likely to be due to the problem related to a high P. vivax background. For P. falciparum, SYBR Green-I method has been successfully applied for in vitro monitoring of sensitivity of the parasite to antimalarial drugs[10]. The results of this malaria SYBR Green I-based fluorescence assay were reported to be in agreement with those of the [3H]ethanoloamine and [3H]hypoxanthine assays. Other fluorescent dyes have also been used for in vitro drug susceptibility assays[17]. PicoGreen has been used and was found to produce results comparable to those of the standard method based on the uptake of [3H]hypoxanthine in P. falciparum isolates[11]. A recently published study compared the PicoGreen method to isotopic and microscopic assays for the measurement of the chloroquine sensitivities of fresh and cryopreserved isolates of P. vivax[18]. Nevertheless, the authors reported no significant difference in the IC50 values regardless of the method used. The authors concluded that the schizont maturation inhibition assay was more reliable than the PicoGreen and isotopic methods to produce valid results. Due to the difficulty in obtaining fresh P. vivax isolate data the best drug susceptibility method should be robust and provide a high success rate. On the basis of this condition and its superior sensitivity, the microscopic method appears better than the SYBR Green-I Green assay for assessing in vitro sensitivity of fresh P. vivax isolates to antimalarial drugs. Further study in a larger number of samples is required to confirm the results.
Acknowledgments
The study was supported by The Commission on Higher Education, Ministry of Education of Thailand and Thailand National Research University (NRU). We thank Ms. Kulaya Ruengweerayut for her kind support for sample collection.
Footnotes
Foundation Project: Supported by the Commission on Higher Education, Ministry of Education of Thailand and Thailand National Research University (NRU).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Moonen B, Cohen JM, Tatem AJ, Cohen J, Hay SI, Sabot O, et al. et al. A framework for assessing the feasibility of malaria elimination. Malar J. 2010;9:322–328. doi: 10.1186/1475-2875-9-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, et al. et al. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8(4):272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 3.Na-Bangchang K, Karbwang J. Current status of malaria chemotherapy and the role of pharmacology in antimalarial drug research and development. Fundam Clin Pharmacol. 2009;23(4):387–409. doi: 10.1111/j.1472-8206.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 4.Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009;22(3):508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD. Effects of chloroquine, quinine, and cycloguanil upon the maturation of asexual erythrocytic forms of two strains of Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1968;17(5):661–671. doi: 10.4269/ajtmh.1968.17.661. [DOI] [PubMed] [Google Scholar]
- 6.Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J. Cultivation of Plasmodium vivax. Trends Parasitol. 2008;24(2):85–88. doi: 10.1016/j.pt.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Tasanor O, Noedl H, Na-Bangchang K, Congpuong K, Sirichaisinthop J, Wernsdorfer WH. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 2002;83(1):49–61. doi: 10.1016/s0001-706x(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 8.Russell BM, Udomsangpetch R, Rieckmann KH, Kotecka BM, Coleman RE, Sattabongkot J. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob Agents Chemother. 2003;47(1):170–173. doi: 10.1128/AAC.47.1.170-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noedl H, Wongsrichanalai C, Wernsdorfer WH. Malaria drug sensitivity testing: new assays, new perspectives. Trends Parasitol. 2003;19(4):175–181. doi: 10.1016/s1471-4922(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 10.Rason MA, Randriantsoa T, Andrianantenaina H, Ratsimbasoa A, Menard D. Performance and reliability of the SYBR Green I based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans R Soc Trop Med Hyg. 2008;102(4):346–351. doi: 10.1016/j.trstmh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, et al. et al. Novel, rapid, and inexpensive cell based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother. 2004;48(5):1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, et al. et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52(3):1040–1045. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasanor O, Ruengweerayut R, Sirichaisinthop J, Congpuong K, Wernsdorfer WH, Na-Bangchang K. Clinical-parasitological response and in-vitro sensitivity of Plasmodium vivax to chloroquine and quinine on the western border of Thailand. Trans R Soc Trop Med Hyg. 2006;100(5):410–418. doi: 10.1016/j.trstmh.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Hastings MD, Maguire JD, Bangs MJ, Zimmerman PA, Reeder JC, Baird JK, et al. et al. Novel Plasmodium vivax dhfr alleles from the Indonesian Archipelago and Papua New Guinea: association with pyrimethamine resistance determined by a Saccharomyces cerevisiae expression system. Antimicrob Agents Chemother. 2005;49(2):733–740. doi: 10.1128/AAC.49.2.733-740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rungsihirunrat K, Na-Bangchang K, Hawkins VN, Mungthin M, Sibley CH. Sensitivity to antifolates and genetic analysis of Plasmodium vivax isolates from Thailand. Am J Trop Med Hyg. 2007;76(6):1057–1065. [PubMed] [Google Scholar]
- 16.Japrung D, Leartsakulpanich U, Chusacultanachai S, Yuthavong Y. Conflicting requirements of Plasmodium falciparum dihydrofolate reductase mutations conferring resistance to pyrimethamine-WR99210 combination. Antimicrob Agents Chemother. 2007;51(12):4356–4360. doi: 10.1128/AAC.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48(5):1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaisavee V, Suwanarusk R, Nosten F, Kyle DE, Barrends M, Jones J, et al. et al. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp Parasitol. 2006;114(1):34–39. doi: 10.1016/j.exppara.2006.02.006. [DOI] [PubMed] [Google Scholar]