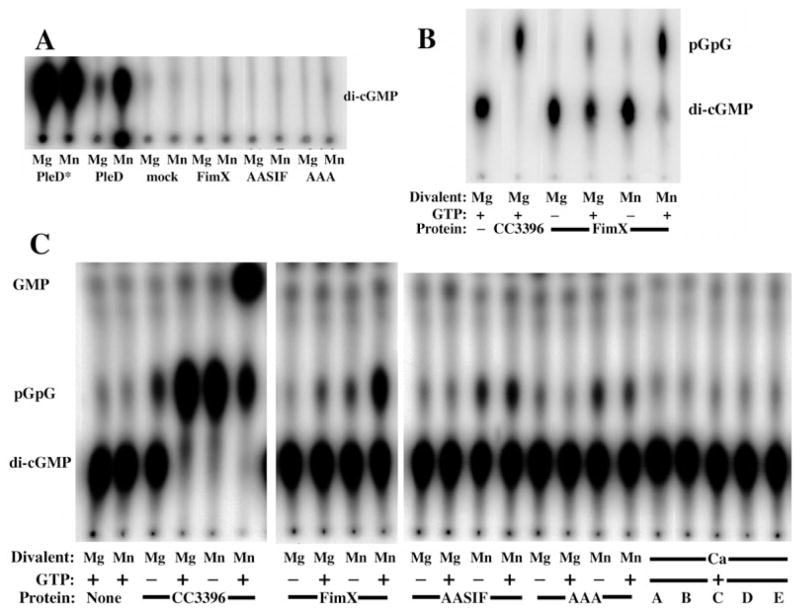
Fig. 5. FimX exhibits phosphodiesterase activity which is stimulated by GTP, but has no detectable DGC activity.
A. TLC of DGC assay products. Purified hexahistidine-tagged proteins were assayed in the presence of 10 mM MgCl2 or MnCl2 (as indicated) for the ability to synthesize c-di-GMP from GTP. Reactions were stopped after 30 min by the addition of 0.5 M EDTA and assayed by TLC on PEI-cellulose as detailed in Experimental procedures.
B. TLC of phosphodiesterase assay products. Purified hexahistidine-tagged proteins were assayed in the presence of 10 mM MgCl2 or MnCl2 (as indicated), 100 μM GTP (as indicated) and [32P]-c-di-GMP for 30 min. Reactions were stopped by the addition of 0.5 M EDTA and assayed by TLC on PEI-cellulose as detailed in Experimental procedures. No product with Rf(pGpG) was detected when substrate was incubated with MgCl2 and GTP in the absence of protein.
C. Phosphodiesterase assay of wild-type FimX and point mutants. Hexahistidine-tagged proteins were incubated with [32P]-c-di-GMP, 10 mM MgCl2, MnCl2, or CaCl2 (as indicated) and 100 μM GTP (as indicated) for 60 min at 25°C. Reaction products were separated by TLC on PEI-cellulose as above. Lanes A–E correspond to: no protein (A); CC3396 (B); FimX (C); FimX(GDSIF→AASIF) (D); FimX(EVL→AAA) (E).