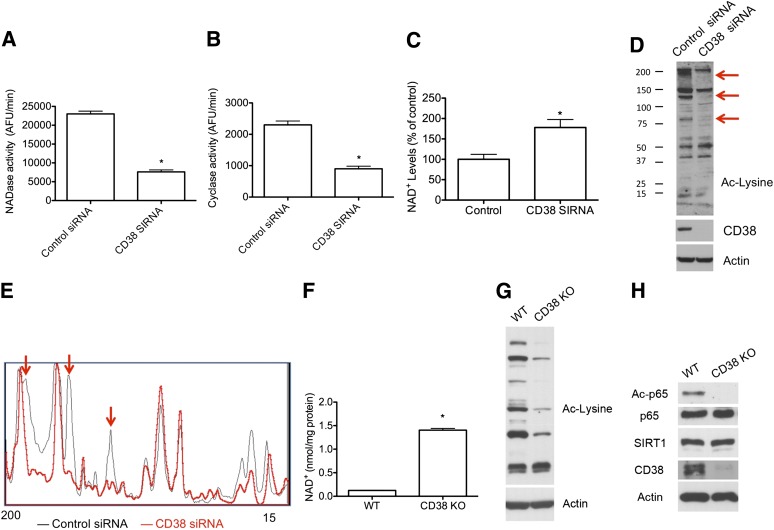
FIG. 2.
CD38 downregulation increases NAD+ and decreases protein acetylation in cells. A549 cells were transfected with a scrambled siRNA (control siRNA) or human CD38 siRNA. After 72 h, cells were harvested and NAD+ase activity (A), ADP-ribosyl-cyclase activity (B), and total intracellular NAD+ levels (C) were measured from cell lysates. *P < 0.05, n = 3. D: Western blot showing total protein acetylation in cells transfected with control siRNA or with human CD38 siRNA. Anti–acetylated (Ac) lysine antibody was used. Red arrows highlight the main bands that showed variations in intensity. E: Intensity profile of the Western blot shown in D. Western blots were scanned and intensity profile was obtained using Image J. Red arrows correspond with intensity of the same bands showed in D. F–H: Primary MEFs were purified and cultured from wild-type (WT) and CD38 knockout (KO) mice. F: Intracellular NAD+ levels (*P < 0.05, n = 3). G: Western blot from wild-type and CD38 knockout MEFs showing total protein acetylation in these cells. H: Representative Western blot in wild-type and CD38 knockout MEFs. Acetylated RelA/p65 (K310), total RelA/p65, SIRT1, CD38, and actin antibodies were used.