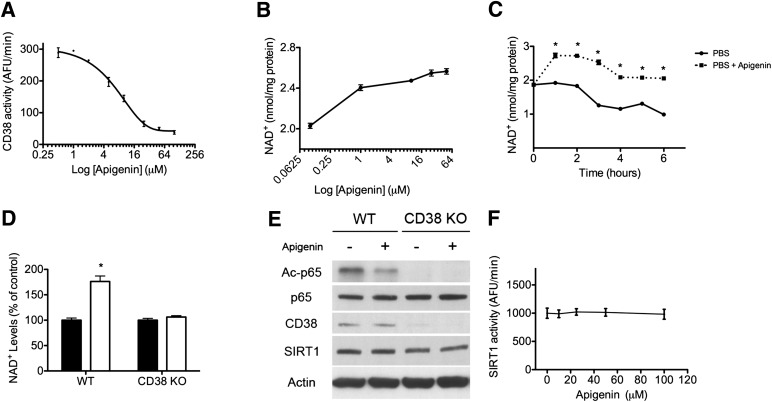
FIG. 5.
CD38 inhibition by apigenin increases NAD+ and decreases protein acetylation in cells. A: Endogenous CD38 NAD+ase activity was measured in protein lysates from A549 cells. Apigenin was used in the 2.5–100 μmol/L concentration range. Each measurement was done in triplicate. Data points were fitted to a standard competitive inhibition curve using a nonlinear regression program (GraphPad Prism) to yield the IC50 value. B: NAD+ dose-response curve in A549 cells treated with apigenin. Cells were incubated with apigenin for 6 h before NAD+ extraction. C: NAD+ time course in A549 cells incubated in PBS (●) or in PBS plus 25 μmol/L apigenin (■) (*P < 0.05, n = 3). D: Intracellular NAD+ levels in wild-type (WT) and CD38 knockout (KO) MEFs treated with vehicle (control) (■) or with apigenin (25 μmol/L) (□) for 6 h. NAD+ levels were expressed as percent change with respect to the control for both cells. Total NAD+ levels were significantly higher in CD38 knockout MEFs. (See Fig. 2F.) *P < 0.05, n = 3. E: Western blot of wild-type and CD38 knockout MEFs that were treated with vehicle or apigenin as described in D. Samples were immunoblotted for acetylated (Ac)-p65 (K310), total p65, CD38, SIRT1, and actin. F: In vitro SIRT1 activity using recombinant-purified human SIRT1. SIRT1 activity was measured in the presence of different concentrations of apigenin (0–100 μmol/L). Activity was determined in the linear portion of the reaction.