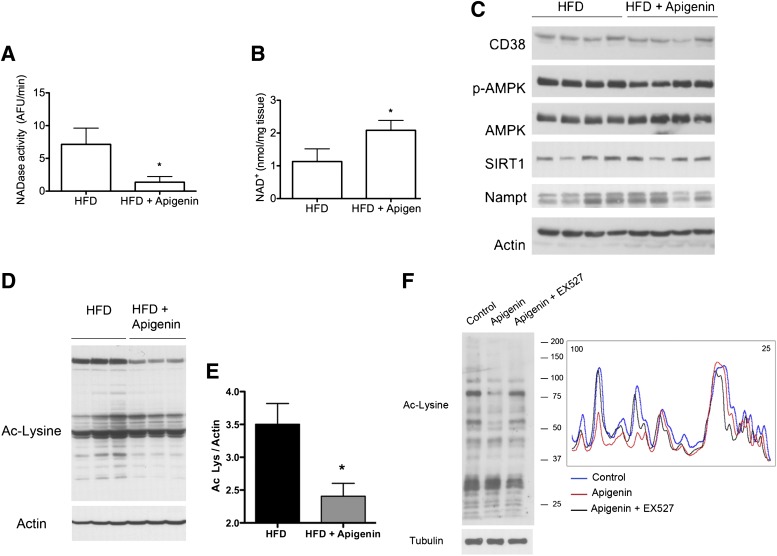
FIG. 6.
CD38 inhibition by apigenin increases NAD+ and decreases protein acetylation in vivo. A–E: Mice were fed a high-fat diet (HFD) for 4 weeks and then split in two groups. One group was injected with apigenin (100 mg/kg i.p.) and the other with vehicle (DMSO) with a single dose daily for 1 week. A: CD38 activity in the liver at the end of the treatment with apigenin (*P < 0.05, n = 6 animals per group). B: NAD+ levels in the liver after the treatment (*P < 0.05, n = 6 animals per group). C: At the end of the treatment, liver samples were obtained and immunoblotted for CD38, phosphorylated (p)-AMPK (Thr172), AMPK, SIRT1, Nampt, and actin. D: Liver samples were immunoblotted for global acetylation of proteins using an anti–acetylated (Ac) lysine (Lys) antibody. Western blots were scanned and an intensity profile was obtained using Image J. The area under the curve is shown. (*P < 0.05, n = 3 per group.) F: Human HepG2 cells were incubated with vehicle (DMSO), apigenin (25 μmol/L), or apigenin plus EX527 (10 μmol/L) for 6 h. Cell lysates were immunoblotted for acetylated lysine to determine total protein acetylation levels (left panel). The intensity profile of the Western blot was obtained using Image J (right panel).