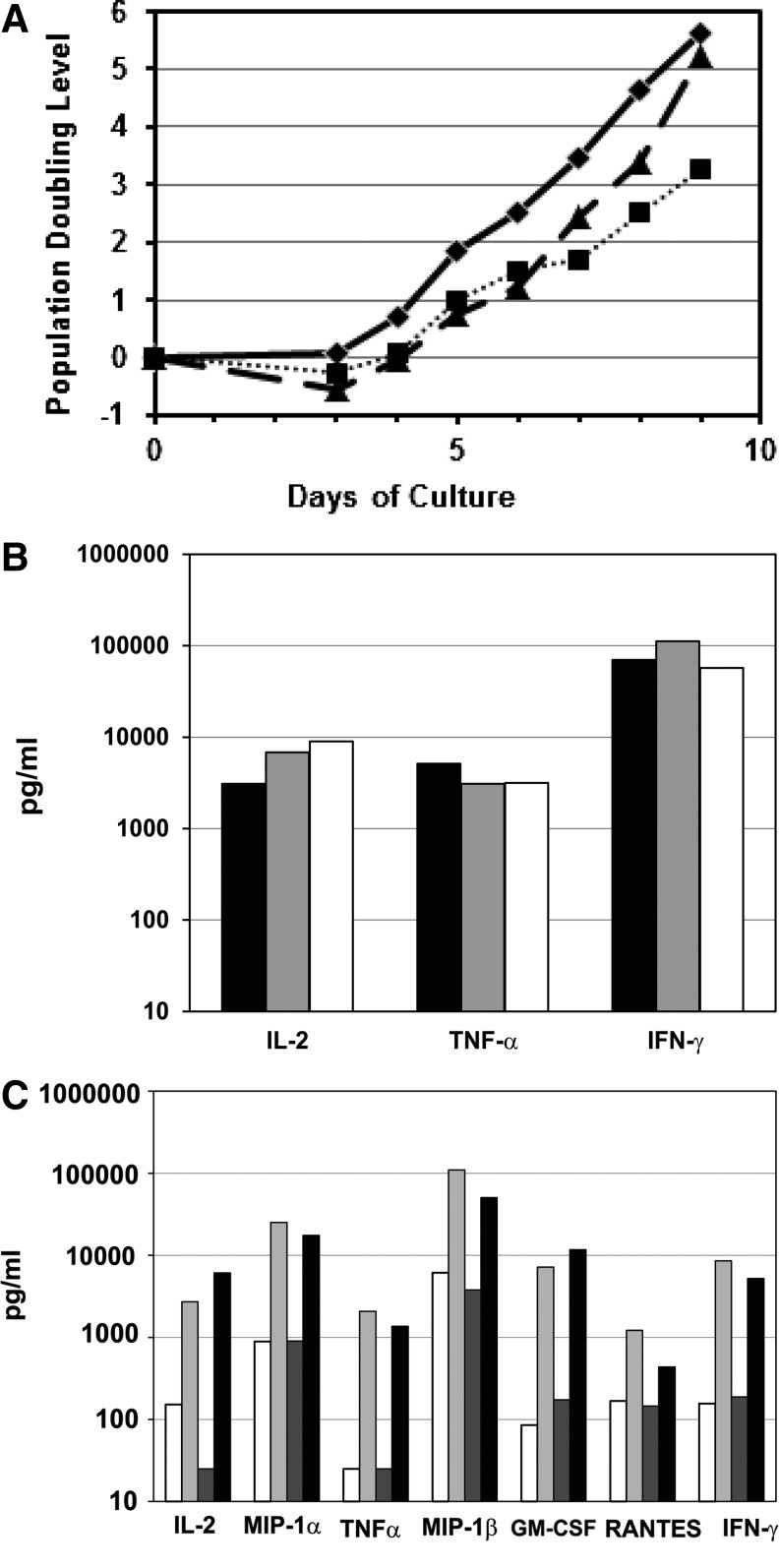
FIG. 4.
Validation of large-scale ex vivo clinical process for T-cell expansion and CCR5 modification incorporating a dynamic perfusion bioreactor. (A) Cells from an HIV-1 infected donor were stimulated ex vivo and transduced as described in the Methods section. Research scale untransduced, solid line and diamond symbols; research scale transduced, dotted line and square symbols; clinical scale transduced, dashed line and triangle symbols. (B) The production of IL-2, TNF-α, and Interferon-γ in supernatants are shown—research scale untransduced, research scale CCR5-ZFN transduced, and clinical scale CCR5-ZFN-transduced cultures. Expanded T cells from the culture in (A) were harvested, washed, resuspended in fresh media, and restimulated with fresh anti-CD3/CD28 mAb-coated beads. Supernatants were collected 24 hr later. Research scale untransduced, black bars; research scale transduced, gray bars; clinical scale transduced, white bars. An additional HIV-1 infected donor (C) was compared for production of cytokines as well as β-chemokines as in (B). Spontaneous secretion of cytokines and β-chemokines (production in the absence of restimulation) was also assayed as a measure potency of final product cells for infusion. Cells were harvested at the end of culture, and beads were removed and resuspended in fresh culture media. Supernatants were collected 24 hr later. Shown is the production of IL-2, TNF-α, MIP-1α, MIP-1β, GM-CSF, RANTES, and Interferon-γ. Research scale untransduced and unstimulated (spontaneous), white; research scale untransduced and restimulated with fresh anti-CD3/anti-CD28 mAb beads, light gray; clinical scale transduced and unstimulated (spontaneous), dark gray; clinical scale transduced and restimulated with fresh anti-CD3/anti-CD28 mAb beads, black.