Abstract
Objective
Fetal dysregulation of T helper (Th) cell pathways may predispose to allergy, as high cord blood Th2/Th1 ratios have been shown to precede development of allergic diseases. We aimed to determine whether prenatal eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation reduces Th2 to Th1-associated chemokine ratios. We also explored the effect of mode of delivery on Th2/Th1 ratios.
Study design
We conducted a secondary analysis of a randomized placebo controlled trial initially performed to assess the effects of DHA or EPA supplementation on pregnancy-related depressive symptoms among 126 participants. Cord plasma specimens from 98 newborns were assayed for chemokines associated with Th2 [TARC (CCL17), MDC (CCL22), Eotaxin (CCL 11)] and Th1 [IP10 (CXCL 10)] by ELISA and Multiplex immunoassays. Ratios of log-transformed chemokines MDC/IP10 and TARC/IP10 were compared between groups by ANOVA. Multiple linear regression was performed to examine associations between treatments and chemokine ratios, adjusting for covariates.
Results
After adjusting for gestational age at delivery, birth weight and mode of delivery, both omega-3 supplementation groups were associated with lower MDC/IP10 ratios than placebo [EPA: coefficient −1.8 (95% CI −3.6, −0.05), p=0.04; DHA: −2.0 (95% CI −3.9, −0.07), p=0.04]. Similar associations were found for TARC/IP10 [EPA: −1.5 (95% CI −3.0 0.06), p=0.06; DHA −2.2 (95% CI −3.8,−0.52), p=0.01]. Cesarean delivery was associated with higher MDC/IP10 [1.6 (95% CI 0.01, 3.3), p=0.049] and TARC/IP10 [1.5, (95%CI 0.1, 2.9), p=0.042] ratios than vaginal delivery.
Conclusions
Prenatal supplementation with EPA and DHA resulted in decreased cord blood Th2/Th1 chemokine ratios. Cesarean delivery was associated with a pronounced Th2 deviation at birth.
Keywords: fetal, programming, chemokines, allergy, omega-3
INTRODUCTION
Allergic diseases have become an important cause of childhood morbidity in the developed world.1 Since allergic diseases are often diagnosed in early childhood, it has been suggested that etiologic factors may take place in utero or during the neonatal period.2–5 Prenatal factors that have been suggested to predispose to allergic diseases include a maternal diet relatively deficient in omega-3 fatty acids (the Western diet) and cesarean delivery.6,7 One mechanism whereby developmental programming for allergic diseases may take place is through an imbalance of the T helper cell one and two (Th1/Th2) pathways during fetal life.8–10 A plausible mechanism that explains how omega-3 fatty acids alter the T helper balance is through the suppression of interleukin 13 (IL-13) cytokine production, which induces immunoglobulin E (Ig E) synthesis in B cells as well as Th2 differentiation in T cells.11 Chemokines have a crucial role in establishing the Th1/Th2 balance and are consider useful markers of immunity. Higher levels of Th2-associated chemokines as well as higher ratios of Th2- to Th1-associated chemokines in umbilical cord blood have been reported to precede allergy development.12–14
Recent research has focused on prenatal dietary supplements as potential primary preventive interventions that might decrease risk for allergic diseases. A recent meta-analysis of trials of prenatal supplementation suggested that prenatal supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) might reduce risk for asthma and positive skin prick test to egg,15 while a large randomized controlled trial (N=706) of prenatal DHA supplementation with 800 mg daily found that prenatal supplementation significantly reduced eczema and egg sensitization in the first year of life, although there was no overall effect on immunoglobin E-associated allergic diseases.16 One potential mechanism whereby maternal omega-3 fatty acid supplementation may induce these protective effects in the immune system is by altered expression of umbilical cord chemokines. However, no previous studies have evaluated whether dietary supplementation with omega 3-fatty acids during pregnancy has an impact on the Th1/Th2 balance of circulating chemokines at birth.
We performed this study to evaluate whether Th2 to Th1 associated chemokine ratios [macrophage-derived chemokine (MDC)/interferon-inducible protein-10 (IP10)] and [thymus and activation-regulated chemokine (TARC)/IP10)] are decreased in neonates born to mothers who received prenatal supplementation with EPA-rich fish oil and DHA-rich fish oil compared with those who received soy oil placebo. We likewise sought to evaluate whether levels of Th2 associated chemokines TARC (CCL17), Eotaxin CCL11 and MDC (CCL22) are decreased in neonates born to supplemented mothers. Our secondary aims were to examine the effect of mode of delivery on these same chemokine ratios and to evaluate the effect of supplementation on Th2 and Th1-associated chemokines and their ratios in maternal blood.
METHODS
This research study was a secondary analysis of the Mothers, Omega-3 & Mental Health Study. The parent study was a three arm, prospective, double blind, placebo-controlled, randomized controlled trial designed to assess whether omega-3 fatty acid supplementation during pregnancy may prevent antenatal and postpartum depressive symptoms among pregnant women selected based on risk for depression. Our analysis utilized stored samples collected from the parent study. Details of the overall study design have been published previously.17
Study population
In the parent study, we enrolled 126 pregnant women from prenatal clinics at the University of Michigan Hospital and Health Centers and St. Joseph’s Mercy Hospital/Integrated Health Associates and followed them prospectively through pregnancy and up to 6 weeks postpartum. To qualify for the study, women needed to be at risk for depression, based on (i) a history of major depressive disorder, (ii) a history of postpartum depression, or (iii) an Edinburgh Postnatal Depression Score (EPDS) score between 9 and 19. Participants were enrolled between 12–20 weeks gestation and were randomized to one of three groups: a) EPA-rich fish oil supplement (1060 mg EPA plus 274 mg DHA; contained an approximate 4:1 ratio of EPA to DHA) (intervention #1); b) DHA-rich fish oil supplement (900 mg DHA plus 180 mg EPA; contained an approximate of 4;1 ratio of DHA to EPA) (intervention #2); and c) a placebo (control arm; contained 98% soybean oil and 1% each lemon and fish oil). The aims and primary outcomes of the study have been reported elsewhere.17
The Institutional Review Boards of the University of Michigan Hospital (Ann Arbor, MI) and St. Joseph Mercy Hospital (Ypsilanti, MI) approved the study and written informed consent was obtained from all women.
Baseline clinical and demographic characteristics were collected at enrollment. Maternal allergy history was abstracted by chart review. Maternal allergy was defined by any history of asthma or wheezing, eczema, skin allergy or atopic dermatitis and allergic rhinitis. Maternal family history of allergy was also abstracted.
Chemokine analysis was performed on maternal venous blood samples drawn at enrollment between 12 and 20 weeks gestation, (n= 117), and after supplementation at 34–36 weeks gestation (n=112). Participants were allowed to eat a low fat breakfast on the morning of these study visits; blood was drawn after a 4 hour fast. Umbilical cord blood was collected at delivery using aseptic technique from 98 neonates whose mothers participated in the trial. Blood samples were processed within 12 hours and aliquots of plasma were frozen at −70°C temperatures under argon until analysis. Blood sample analyses from the parent study were performed for baseline, post-supplementation, and cord blood levels of omega-3 fatty acids.
Chemokine analysis
We performed a secondary analysis of stored samples for the chemokines of interest in the following manner. The chemokines TARC (CCL17) and MDC (CCL22) were measured using commercially available Multiplex immunoassays kits (pg/ml; Invitrogen Corporation, Carlsbad, CA; detection threshold 23.46 and 68.3 pg/ml, respectively). Determination of Eotaxin (CCL 11) and IP-10 (CXCL 10) were estimated using enzyme-linked immunoabsorbent assay (ELISA) kits (pg/ml; R&D systems, Minneapolis, MN, USA; detection thresholds 19.2 and 3.84 pg/ml respectively) according to the manufacture’s protocols.
Statistical analysis
Descriptive statistics were computed according to treatment arm, and appropriate transformations were made (eg, log transformation) for variables not following a normal distribution. For comparison of continuous variables between groups within each study visit, ANOVA or two-sample t-tests for independent samples were used; for categorical variables Fisher’s exact test was used. Intention-to-treat analysis was performed. Multiple linear regression was used to examine the association between treatment arm and chemokine levels, while adjusting for covariates of a priori judgment to be relevant, including gestational age, birth weight, and mode of delivery. Gestational age of delivery was considered a potential confounder because pregnancy appears to have three distinct immunologic phases characterized by distinct biologic processes. There is a unique inflammatory or anti-inflammatory environment characterizing each stage of pregnancy. It has been described that first and third trimesters are proinflammatory (Th1), whereas the second trimester represents an anti-inflammatory phase also known as Th2 environment.18 Mode of delivery and birth weight were also included as covariates, as both have been reported to impact immune function.19,20 A probability level of <0.05 was considered to be statistically significant. Statistical analyses were performed using Stata v12 (StataCorp, College Station, TX).
Sample size considerations
This work is a secondary analysis of a randomized controlled trial of 126 subjects that was designed to detect a 50% reduction in depression score between the intervention and control groups. As such, it was not designed to detect possible differences in allergic diseases. Consequently, our evaluation of chemokines and allergic symptoms was exploratory.
RESULTS
Demographics data
Of the 126 women who enrolled in the parent study, 117 completed the trial and had available specimens for this ancillary study. The baseline characteristics of enrolled the participants are detailed in this analysis are detailed in Table 1. No differences in maternal age, ethnicity and mode of delivery were encountered among the 3 groups. Prenatal supplementation with DHA was associated with statistically significant increase in gestational age (GA) at delivery with a one-week prolongation of pregnancy when compared with the EPA and placebo groups (Table 2); birth weight was also significantly higher in the DHA group. There were no differences in baseline maternal DHA and EPA levels or in the proportion of participants who reported a personal history of allergic diseases.
Table 1.
Maternal Baseline Characteristics among participants included in secondary analysis (n=117)
| Treatment Arm
|
p-value | |||
|---|---|---|---|---|
| EPA-Rich Fish Oil (n=40) | DHA-Rich Fish Oil (n=37) | Placebo (n=40) | ||
| Age, years | 30.0 ± 4.9 | 30.2 ± 4.7 | 30.7 ± 5.8 | NS |
| Gestational age at enrollment, weeks | 16.1 ± 2.6 | 16.8 ± 2.3 | 16.2 ± 2.4 | NS |
| Gravidity | 2.4 ± 1.3 | 2.5 ± 1.2 | 2.6 ± 2.06 | NS |
| Race/ethnicity | NS | |||
| White | 33 (82.5) | 28 (75.7) | 34 (85.0) | |
| African-American | 4 (10.0) | 5 (13.5) | 1 (2.5) | |
| Hispanic/Latina | 1 (2.5) | 3 (8.1) | 2 (5.0) | |
| Asian | 1 (3) | 1 (3) | 1 (2) | |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 1 (2) | |
| Native Hawaiian or other Pacific Islander | 1 (2.5) | 0 (0) | 0 (0) | |
| History of allergy (self-reported) | 8 21) | 9 (24) | 9 (22) | NS |
Data are expressed as mean ± standard deviation or frequency (%).
SD = standard deviation, NS= not significant.
Table 2.
Birth outcomes among neonates with cord blood specimens analyzed in this study (n=98)
| Treatment Arm
|
p-value | |||
|---|---|---|---|---|
| EPA-Rich Fish Oil (n=32) | DHA-Rich Fish Oil (n=32) | Placebo (n=34) | ||
| Gestational age at delivery | 39.2 ± 1.3 | 40.1 ± 1.4 | 39.1 ± 1.5 | <0.01 |
| Birth weight (gm) | 3480 ± 536 | 3749 ± 469 | 3289 ± 569 | <0.01 |
| Mode of delivery | NS | |||
| Cesarean section | 8 (25.0) | 11 (34.4) | 11 (32.4) | |
| Spontaneous vaginal delivery | 23 (71.9) | 21 (65.6) | 22 (64.7) | |
| Operative vaginal | 1 (3.1) | 0 (0) | 1 (2.9) | |
Data are expressed as mean ± SD or frequency (%).
NS not significant
Maternal chemokines and chemokine ratios
No differences in the levels of chemokines (IP-10, MDC, TARC and Eotaxin) were detected at baseline or after the intervention. There was no significant difference in the chemokine levels between the placebo, EPA, and DHA treated groups.
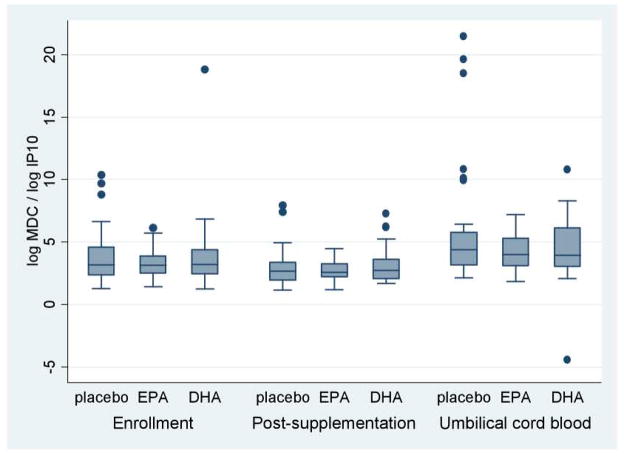
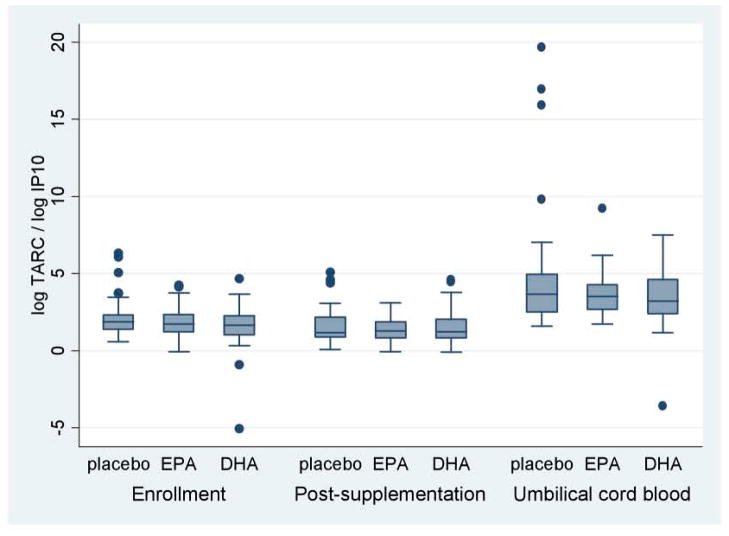
There were no differences detected in the unadjusted mean maternal chemokine ratios MDC/IP10 and TARC/IP10 between the DHA, EPA and placebo groups at each visit (Figures 1 and 2, Table 3).
Figure 1. Ratios MDC/IP10 chemokines.
This figure presents the unadjusted macrophage derived chemokine (MDC)/interferon inducible protein 10 (IP 10) ratios according to treatment group, i.e. EPA-rich fish oil (EPA), DHA-rich fish oil (DHA), or placebo. Ratios are presented from maternal plasma before and after supplementation and from umbilical cord blood.
Figure 2. Ratios of TARC/IP10 chemokines.
This figure presents the unadjusted thymus and activation-regulated chemokine (TARC) /interferon inducible protein 10 (IP 10) ratios according to treatment group, i.e. EPA-rich fish oil (EPA), DHA-rich fish oil (DHA), or placebo. Ratios are presented from maternal plasma before and after supplementation and from umbilical cord blood.
Table 3.
Ratios of Th2 to Th1 associated chemokines in maternal blood at enrollment and post supplementation, as well as umbilical cord blood.
| Enrollment n=117
|
Post-supplementation n=112
|
Umbilical cord blood n=98
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPA | DHA | Placebo | P | EPA | DHA | Placebo | P | EPA | DHA | Placebo | P | |
| log MDC/logIP-10 | 3.2 ± 1.2 | 3.3 ± 4.4 | 3.8 ± 2.1 | NS | 2.7 ± 0.8 | 3.2 ± 1.4 | 3.0 ± 1.4 | NS | 4.3 ± 1.5 | 4.5 ± 2.7 | 5.9 ± 4.9 | NS |
| log TARC/log IP-10 | 1.8 ± 1.0 | 1.7 ± 1.6 | 2.1 ± 1.3 | NS | 1.4 ± 0.8 | 1.6 ± 1.2 | 1.6 ± 1.2 | NS | 3.7 ± 1.6 | 3.4 ± 2.1 | 4.9 ± 4.4 | NS |
EPA = EPA-rich fish oil group
DHA = DHA-rich fish oil group
MDC (CCL22) macrophage-derived chemokine
IP10 (CXCL10) interferon-inducible protein-10
TARC (CCL17) thymus and activation-regulated chemokine
NS not significant
Data expressed as mean ± standard deviation.
Umbilical cord plasma chemokines and chemokine ratios
Chemokines were assayed from the cord blood of 98 infants. We found no differences in cord plasma IP-10, MDC, TARC and Eotaxin between the treatment groups. Ratios of log MDC/logIP10 were lower in the EPA (4.3 ± 1.5) and DHA (4.5 ± 2.7) arms compared to placebo (5.9 ± 4.9), a trend which bordered significance for EPA versus placebo (p=0.08). Similarly, ratios of log TARC/log IP10 were lower in the EPA (3.7 ± 1.6) and DHA (3.4 ± 2.1) groups compared to placebo (4.9 ± 4.4), bordering on significance for DHA versus placebo (p=0.08) (See Table 3).
After adjusting for gestational age at delivery, birth weight, and mode of delivery, both EPA and DHA arms were associated with significant reductions in ratios of log MDC/log IP-10 compared to placebo [EPA: β= −1.8 (95% CI −3.6, −0.05), p=0.04; DHA: −2.0 (95% CI −3.9, −0.07), p=0.04]. Further, cesarean delivery was independently associated with increased log MDC/log IP-10 compared to spontaneous vaginal delivery, in the multivariable model [β=1.6 (95% CI 0.01, 3.3), p=0.049].
Similar associations were found for TARC/IP-10 ratios in umbilical cord blood. After adjusting for gestational age at delivery, birth weight and mode of delivery, the logTARC/logIP-10 ratios in the DHA group were significantly lower than placebo [−2.2 (95% CI −3.8, −0.52), p=0.01] and trended towards significance for EPA [−1.5 (95% CI −3.0, 0.06), p=0.06]. Again, cesarean delivery compared to spontaneous vaginal delivery was significantly associated with higher ratios [1.5 (95% CI 0.05, 2.9), p=0.042].
Umbilical cord levels of DHA and EPA
In babies born to mothers who had received DHA-rich fish oil, umbilical cord serum concentrations of DHA (expressed as percent of total serum fatty acids) were significantly higher (7.4 ± 2.8) compared to those from babies born to mothers who received EPA-rich fish oil (5.9 ± 2.0) and placebo (5.2 ± 2.1 arms p=0.007). Umbilical cord serum concentrations of EPA were higher in the EPA rich fish oil group (0.61 ± 0.5) than in the DHA (0.56 ± 0.4) and placebo (0.55 ± 0.5) arms, though these differences did not reach statistical significance.
COMMENT
The principal finding of this study was that prenatal EPA-and DHA-rich fish oil supplementation modulates fetal the Th2/Th1 balance in cord blood. Similarly, cesarean delivery also has a significant effect on Th2/Th1 ratios at birth. These findings may help to explain the results of prior epidemiologic studies that found that maternal diet and mode of delivery may significantly increase risk for allergic disease.6–7
Conversely, our study found no significant effect of supplementation on maternal chemokine profiles. This finding is consistent with the results of a recent systematic review of the effect of dietary omega-3 fatty acid supplementation on inflammatory biomarkers including chemokines CCL2, CCL3, CCL5 and CCL11.21 The authors found that supplementation has no effect on these markers in healthy volunteers.21
A strength of this study was its randomized, double blind study design, as well as its analysis by intent to treat. Based on the randomized design, there were no significant differences between subjects in the three groups in past history of allergic diseases. The chemokine outcome measure, although a proxy for risk for allergic disease, is also free of risk for recall bias.
A weakness of our study is that it is a secondary analysis and our subjects were not selected based on predisposition to allergic disease. However, our subjects were selected based on predisposition to perinatal depressive symptoms, which other investigators shown to be associated with aberrant activation of the inflammatory response.22 In non-pregnant individuals, Grass-Oliveri, et al.,23 have shown that major depressive disorder and suicidal ideation are associated with increased levels of the TH2-associated chemokine, Eotaxin. Finally, Mattes, et al.,24 have shown that mild to moderate maternal depressive symptoms in mid pregnancy (BDI>10) are associated with a generalized activation of the fetal (cord blood) immune response, However, whether maternal depressive symptoms predispose the offspring to allergic diseases is currently unknown.
At present the mechanism whereby maternal omega-3 fatty acid supplementation modulates the fetal immune response is not known. One potential explanation of how omega-3 fatty acids may exert the observed effect is through competitive inhibition with omega-6 fatty acids in the formation of eicosanoids.25 In particular, the omega-6 fatty acid, arachidonic acid, is the metabolic precursor of prostaglandin E2, an eicosanoid that reduces production of Th1-associated cytokines and increases production of Th2-associated cytokines.25 By contrast, EPA and DHA are the metabolic precursors of anti-inflammatory eicosanoids.25 EPA and DHA are also the metabolic precursors of resolvins and protectins, specialized pro-resolving lipid mediators that have been demonstrated to reduce pro-inflammatory cytokines in animal models of allergic airway disease.25 However the effect of resolvins and protectins on Th2- and Th1-associated chemokines is unknown at present.
No data have been reported on influence of mode of delivery on the chemokines ratios. Cytokines and chemokines, in particular those with proinflammatory properties (Th1), play an important role during labor and full or preterm delivery that may alter the immunity of the fetus/neonate through regulation of many biological functions. The mode of delivery has been thought to be crucial for establishing the infant’s intestinal microflora. Cesarean delivery may alter the immunity of the fetus/neonate due to lack of intestinal flora leading to sustained Th2 responsiveness supporting the B cell Ig E production.26, 27
Because our study does not have data regarding clinical outcomes of allergic diseases in children born to mothers who participated in our study, more research is necessary before prenatal omega-3 fatty acid supplementation can be recommended for primary prevention of allergic diseases. More research is also needed to explore the molecular mechanisms that underlie the observed modulation of the fetal immune response.
Acknowledgments
The authors would like to acknowledge the contributions of Timothy R. B. Johnson, MD and Cosmas Van de Ven, MD, from the Department of Obstetrics and Gynecology, University of Michigan. Neither Dr. Johnson or Van de Ven received any funding or financial compensation for this work. Ms. Julie C. Chilimigras, Ms. Lucy J. Allbaugh, Ms. Bethany Baker, and Ms. Susan E Hamilton all participated in subject recruitment and data entry. Salary support for Ms. Chilimigras, Ms. Allbaugh, Ms. Hamilton and Ms. Baker was provided from the R21 funding of the parent study. Drs. Kristie Keeton and Jennifer Z. Williams recruited subjects for this study at the Integrated Health Associates study performance site. They did not receive any funding or other compensation for this work. Study supplements and placebo were donated by Nordic Naturals Corporation, Watsonville, CA. Nordic Naturals did not participate in the data analysis or interpretation of the results.
Financial support: This project was supported by the NIH R21 AT004166-03S1 (NCCAM), as well as a University of Michigan Clinical Research Initiatives grant and the University of Michigan General Clinical Research Center (now the Michigan Clinical Research Unit). This research was also supported (in part) by the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (P30 CA046592). This project was supported by the NIH R21 AT004166-03S1 (NCCAM), as well as a University of Michigan Clinical Research Initiatives grant and the University of Michigan General Clinical Research Center (now the Michigan Clinical Research Unit). This research was supported (in part) by the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (P30 CA046592). ECS was supported by 1K01ES019909-01 (NIEHS). This project was also supported by the University of Michigan Department of Obstetrics and Gynecology and the Michigan Institute for Clinical Health Research (MICHR).
Footnotes
Reprints will not be available.
Conflict of Interest Statement: Dr. Mozurkewich served as an invited speaker for the Global Organization for EPA and DHA Omega-3 at the Nutracon 2012 Conference in March 2012 and received reimbursement for travel expenses. The other authors have no conflicts of interest.
This study was conducted in Ann Arbor, Michigan, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3:125–32. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC, Krauss-Etschmann S, de Jong EC, et al. Early nutrition and immunity – progress and perspectives. Br J Nutr. 2006;96:774–90. [PubMed] [Google Scholar]
- 3.Jones CA, Holloway JA, Warner JO. Does atopic disease start in foetal life? Allergy. 2000;55:2–10. doi: 10.1034/j.1398-9995.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 4.Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids. 2007;42:801–10. doi: 10.1007/s11745-007-3030-z. [DOI] [PubMed] [Google Scholar]
- 5.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000;105:S493–8. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 6.Lumia M, Luukkainen P, Tapanainen H, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol. 2011;22:827–35. doi: 10.1111/j.1399-3038.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 7.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–79. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaub B, Liu J, Hoppler S, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol. 2008;121:1491–9. 99 e1–13. doi: 10.1016/j.jaci.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Ohshima Y, Yasutomi M, Omata N, et al. Dysregulation of IL-13 production by cord blood CD4+ T cells is associated with the subsequent development of atopic disease in infants. Pediatr Res. 2002;51:195–200. doi: 10.1203/00006450-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Neaville WA, Tisler C, Bhattacharya A, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol. 2003;112:740–6. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 11.Heinzmann A, Mao XQ, Akaiwa M, et al. Genetic variants of IL-13 signaling and human asthma and atopy. Hum Mol Genet. 2000;9:549–59. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 12.Sandberg M, Frykman A, Ernerudh J, et al. Cord blood cytokines and chemokines and development of allergic disease. Pediatr Allergy Immunol. 2009;20:519–27. doi: 10.1111/j.1399-3038.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 13.Abelius MS, Ernerudh J, Berg G, Matthiesen L, Nilsson LJ, Jenmalm MC. High cord blood levels of the T-helper 2-associated chemokines CCL17 and CCL22 precede allergy development during the first 6 years of life. Pediatr Res. 2011;70:495–500. doi: 10.1203/PDR.0b013e31822f2411. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson TR, Abelius M, Forsberg A, Bjorksten B, Jenmalm MC. A Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization. Clin Exp Allergy. 2011;41:1729–39. doi: 10.1111/j.1365-2222.2011.03827.x. [DOI] [PubMed] [Google Scholar]
- 15.Klemens CM, Berman DR, Mozurkewich EL. The effect of perinatal omega-3 fatty acid supplementation on inflammatory markers and allergic diseases: a systematic review. BJOG. 2011;118:916–25. doi: 10.1111/j.1471-0528.2010.02846.x. [DOI] [PubMed] [Google Scholar]
- 16.Palmer DJ, Sullivan T, Gold MS, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ. 2012;344:e184. doi: 10.1136/bmj.e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozurkewich E, Chilimigras J, Klemens C, et al. The mothers, Omega-3 and mental health study. BMC Pregnancy Childbirth. 2011;11:46. doi: 10.1186/1471-2393-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ly NP, Ruiz-Perez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, Delaney ML, DuBois AM, Levy H, Gold DR, Ryan LM, Weiss ST, Celedon JC. Mode of delivery and cord blood cytokines: a birth cohort study. Clin Mol Allergy. 2006;26:4–13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J, McGuinness OP. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2012 Dec 26; doi: 10.1152/ajpendo.00266.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 22.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23(6):750–54. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012;34(1):71–5. doi: 10.1590/s1516-44462012000100013. [DOI] [PubMed] [Google Scholar]
- 24.Mattes E, McCarthy S, Gong G, van Eekelen JA, Dunstan J, Foster J, Prescott SL. Maternal mood scores in mid-pregnancy are related to aspects of neonatal immune function. Brain, Behavior, and Immunity. 2009;23(3):380–88. doi: 10.1016/j.bbi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012;99:57–67. doi: 10.1016/j.prostaglandins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Bocci V. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med. 1992;35:251–60. doi: 10.1353/pbm.1992.0004. [DOI] [PubMed] [Google Scholar]
- 27.Kero J, Gissler M, Grönlund MM. Mode of delivery and asthma -- is there a connection? Pediatr Res. 2002;52:6–11. doi: 10.1203/00006450-200207000-00004. [DOI] [PubMed] [Google Scholar]