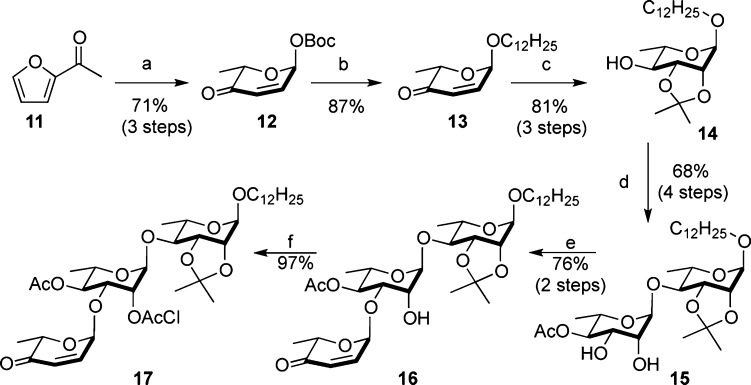
Scheme 2. Approach to the Intermediate 17.
Reagents and conditions: (a) (i) Noyori (S,S), HCO2Na(aq), 10 mol % CTAB, rt, 24 h; (ii) NBS, THF/H2O (3:1), NaHCO3, NaOAc·3H2O, 0 °C, 1 h; (iii) Boc2O, DMAP, CH2Cl2, −78 °C, 12 h. (b) HOCH2(CH2)10CH3, 5 mol % Pd(PPh3)2, CH2Cl2, 0 °C. (c) (i) NaBH4, CeCl3, CH2Cl2/MeOH, −78 °C, 2 h; (ii) OsO4, NMO, t-BuOH/acetone, rt, 12 h; (iii) TsOH, 2,2-DMP, acetone, 0 °C, 4 h. (d) (i) 12, 5 mol % Pd(PPh3)2, CH2Cl2, 0 °C, 2 h; (ii) NaBH4, CH2Cl2/MeOH, −78 °C, 2 h; (iii) Ac2O, Py, rt, 3 h; (iv) OsO4, NMO, t-BuOH/acetone, rt, 12 h. (e) (i) n-Bu2SnO, MeOH, reflux, 1h; (ii) 12, 5 mol % Pd(PPh3)2, CH2Cl2, 0 °C, 2 h. (f) (ClAc)2O, Py, 3 h. Pd(PPh3)2 = Pd2(dba)3·CHCl3/4PPh3.