Abstract
Arteries and veins have been historically defined by the direction of blood flow and oxygen tension within the vessel, in addition to their functional, hemodynamic, and anatomical differences. It is now known that the molecular identity of these vessels is genetically predetermined, with specific molecular pathways activated during the development of arteries and veins. Eph-B4 is a determinant of venous differentiation and Ephrin-B2 is a determinant of arterial differentiation. Placement of a vein into the higher pressure and flow of the arterial circulation results in adaptation of the vein to the arterial environment. There is selective loss of Eph-B4 expression without induction of Ephrin-B2 expression during vein graft adaptation. These findings suggest that loss of venous identity is the crucial mechanism in vein graft adaptation and that developmentally critical determinants of vessel identity are plastic during adult life.
Keywords: arterialization, Notch, Ephrin-B2, Eph-B4, venous identity
History
Recognition of the differences between arteries and veins can be found in Huangdi nejing suwen (The Yellow Emperor's Manual of Corporeal Medicine).1) This historical document described the ancient concept of two separate circulations of body fluids; blood, pumped by the heart and then continuously through the arteries, veins and capillaries, and Ch'i, an ethereal form of energy.1) Ishinpo, the oldest surviving Japanese medical text, was written by Yasuyori Tamba in 984, and describes basic vascular anatomy and circulation. Kaitai-shinsho is the oldest and most famous Japanese anatomy text; it was written by Genpaku Sugita in 1774 and contains detailed anatomical figures of arteries and veins, with early evidence of influence by Western medicine on Japanese medicine.2)
Outside of Asia, the first known documentation of basic circulation was in Greece, approximately 500 BC; Alcmaeon of Croton, an early pioneer of animal dissection, observed arteries and veins to be distinctive structures.3) Hippocrates (460–370 BC), considered the founder of Western medicine, was the first to propose that blood travels in a circle. Using human cadaver dissections, Herophilus of Chalcedon (300 BC) elaborated on the distinction between arteries and veins and discovered that arteries contained blood rather than air.3)
Claudius Galenus of Pergamum (129–216 AC) elevated the earlier Hippocratic concepts to scientific levels through observation of animal dissection and experimentation. He confirmed that arteries contained blood and that arteries and veins were distinct, each with a different type of blood. Galen believed that nutritive blood was made by the liver and carried through veins to the organs where it was expended, and that vital blood was made by the heart and pumped through arteries to carry spirits. He claimed that blood flowed through the heart via interventricular septal pores and that arteries had innate pulsatility, rather than being pumped by the heart. Unfortunately he failed to complete the “circuit” of circulation, having not yet identified the pulmonary circulation.3) In the mid-1200's Ibn al-Nafis, an Arab physician, discovered and described the pulmonary circulation.3)
William Harvey (1578–1657) is credited with the discovery of circulation. He determined that the amount of blood circulating through the body far exceeded that which the body could make and that blood flows through both the systemic and pulmonary circulations. His popular experiment published in Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus (1628) proved that blood in veins moved readily towards the heart but not in the opposite direction.3) The presence of capillaries was discovered after Harvey's death by Marcello Malpighi, using the newly invented microscope of Dutch scientist Antony van Leeuwenhoek (1632–1723).3-5)
Blood Vessel Development
All tissues require the hematogenous transfer of nutrients for survival. The human embryo begins to develop a vascular system by the third gestational week—the mouse by about day 7—when diffusion can no longer meet the embryo's nutritional needs.6-8) Two distinct vascular processes are recognized, each forming new endothelium-lined vessels: vasculogenesis and angiogenesis. Vasculogenesis is the initial embryonic de novo formation of blood vessels, a unique process in which a population of mesodermally derived endothelial cell precursors, the angioblasts, differentiate and assemble into a reticulum of uniformly sized primitive blood vessels known as the primary vascular plexus.9) For example, factors such as vascular endotherial growth factor (VEGF), GM-CSF, and bFGF, and receptors such as VEGFR, neuropilin-1 and Tie-2, are thought to be primary mediators of this process.10, 11)
In the adult, new vessels are produced only through angiogenesis.11) Angiogenesis is the formation of new blood vessels from existing vessels, and is thought to be mediated by factors such as VEGF, Ang1, PDGF-BB, and TGF-β1, as well as the VEGFR, PDGF-βR, Tie-2, and TGF-βR receptors.11) It is a complicated, but well-organized process, requiring modulation of multiple endothelial cell functions such as sprouting and branching.12) On the other hand, arteriogenesis describes the remodeling of small interconnecting arterial anastomoses (collateral formation) with almost no net change in blood flow; this process is triggered by physical stresses that activate the endothelium, such as hypoxia.13, 14)
Blood Vessel Composition
Arteries and veins are anatomically distinct in the adult circulatory system.15) Arteries, exposed to high pressure and flow, have a large diameter with a thick, rigid wall and a small lumen. Arteries transport oxygenated blood away from the heart and contract and relax in response to the sympathetic nervous system. Conversely, veins are a low pressure blood reservoir that return blood to the heart. Veins have greater cross sectional area at any given level with slower flow. Veins are often paired structures and those in the legs typically contain valves. Interestingly, venous portal circulations, such as the mesenteric or pituitary portal circulations, have a second capillary bed.
All blood vessels have the same three-layer wall histological structure comprised of an adventitia, a media and an intima. There are three structurally based categories of arteries: elastic arteries (average diameter of 1 cm), muscular arteries (0.5 mm–1 cm) and arterioles (less than 0.03 cm in diameter). The intima consists of a single layer of endothelial cells lining the lumen of the vessel with an underlying basement membrane of connective tissue. The internal elastic lamina separates the intima from the media. The media is composed largely of smooth muscle cells and a variable amount of connective tissue; the number of concentric smooth muscle layers, and media thickness, increases with increasing artery diameter. The external elastic lamina separates the media from the adventitia. The adventitia is primarily composed of loose connective tissue made up of largely fibroblasts and collagen fibers; the adventitia layer comprises between 10% and 50% of the thickness of arterial walls. The intima of a vein is also composed of endothelium with little or no subendothelial connective tissue. However, venous media have a relatively thin layer of circular smooth muscle cells that may contain collagen and some fibroblasts. Venous adventitia is the thickest structural layer of the vessel and is made up of longitudinal bundles of collagen and elastic fibers with only a few smooth muscle cells.16)
Molecular Fingerprints of Vessels
Arteries and veins are defined in the adult by their functional and anatomical differences. However, the molecular differences between arteries and veins are established before the onset of circulation.17) Molecular differences between arterial and venous endothelial cells have been demonstrated during embryonic development in the chick, mouse and zebrafish.10, 17, 18)
There are several critical molecular determinants of blood vessel development, some of which are receptors that interact with their ligands; others are intracellular signaling molecules or transcription factors; others are likely to be discovered. For example, arteries contain Ephrin-B2, Dll4, CD44 and Neuropilin-1, while veins contain Eph-B4, Neuropilin-2 and COUP-TFII.19–21) Other important receptor-ligand interactions involve Notch, VEGF, angiopoietins and their cognate receptor-Tie-2, FGF and PDGF. Segal et al. demonstrated that while Cav-1 is present throughout the vasculature, Cav-3 is found on arterial but not venous vasculature.22)
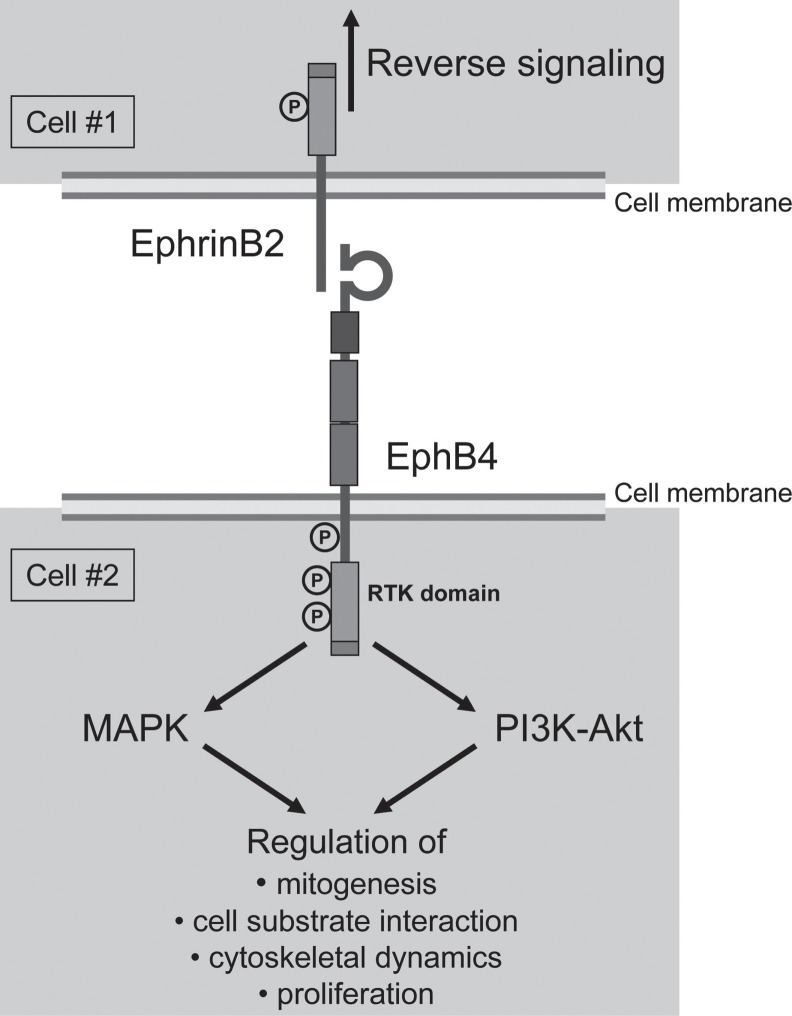
The Eph receptors comprise the largest of the 14 families of receptor tyrosine kinases in mammals and are activated by ligands of a similarly large Ephrin family. Ephrin-Eph signaling was first documented in the nervous system regarding axonal repulsion.23, 24) The transmembrane Eph receptor has a cyoplasmic kinase domain that is activated upon binding with the Ephrin ligand's extracellular globular domain (Fig. 1). Unlike the majority of receptor tyrosine kinases, bidirectional signaling can originate from both the Ephrin ligand and the Ephrin receptor. The membrane spanning region with Ephrins enables these ligands to potentially transmit signals into the “ligand” cell, in what has been termed “reverse” signaling.25)
Fig. 1.
Model of the Eph receptors and Ephrins. Ephrin-expressing cell #1 (top) binding with Eph-expressing cell #2 (bottom). Forward and reverse signaling both play an important role in vascular formation and function.
After their initial discovery as orphan receptors by Bennett et al. in 1995, Eph receptors were found to have matching Ephrin ligands and early research efforts determined that Ephrins and Ephs participate in developmental processes.26) Eph receptors are divided into two subclasses: EphA and EphB. It was originally assumed that Ephrin subclasses A and B only interacted with their own respective Eph receptor, but it has been found that the binding preference of Ephrins is promiscuous and spans classes.23, 27, 28) The Ephrin-A subclass is linked to the membrane via a glycosylphosphatidylinositol (GPI) linkage, while members of the Ephrin-B subclass are transmembrane proteins.29, 30) Remarkably, in contrast to other ligands that are uniformly expressed throughout the circulatory system, Ephrin-B2 was found to be specifically expressed only by arteries while Eph-B4, one of its receptors, is expressed only on veins.17, 31, 32) These data provided one of the first examples of a genetic distinction between these two vessel subtypes and suggested that Ephrin-mediated interactions may be essential for angiogenesis.33)
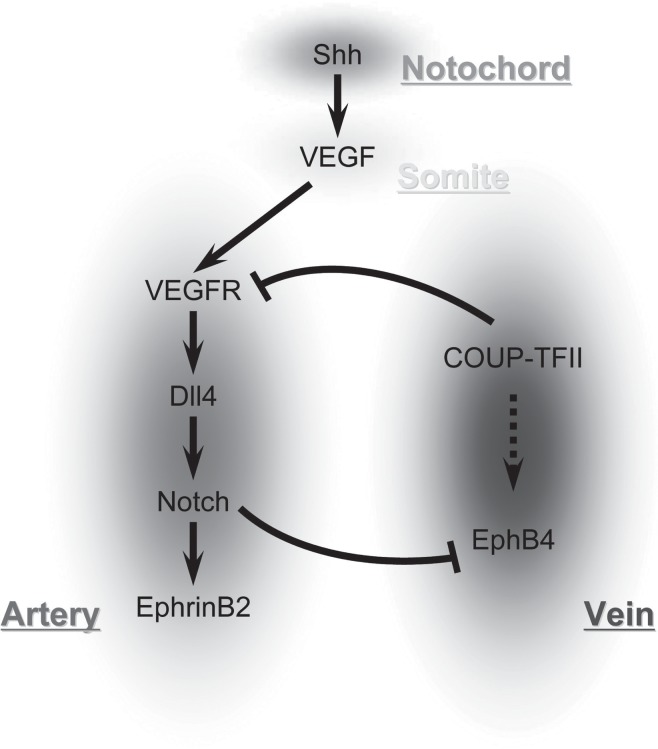
Ephrin-Eph interactions show that differences between arteries and veins are genetically predetermined; this was suggested since the knockout of either Ephrin-B2 or Eph-B4 produced similar significant cardiovascular defects, including disruption of angiogenesis of the yolk sac in mice.17) Both Ephrin-B2 and Eph-B4 are factors required for normal vessel development; potential regulatory mechanisms of the Ephrins include Notch, VEGF, and sonic hedgehog (Shh) (Fig. 2).
Fig. 2.
Signaling pathways that control arterial-venous differentiation.
VEGF is known to elicit a wide range of responses in endothelial cells such as proliferation, migration and survival and is critical in neovascularization.34, 35) VEGF-A lies upsteam of the Notch signaling pathway and upregulates Notch-1 and Dll4 in arterial endothelial cells (Fig. 2).18) Binding of VEGF-A to its heterodimeric receptor activates the Notch signaling pathway in endothelium. VEGF-A acts downstream of Shh and upstream of Notch to determine arterial fate.
Sonic hedgehog induces arterial differentiation and thus is critical for blood vessel development; embryos lacking Shh activity fail to undergo arterial differentiation (Fig. 2).18, 36) Recent work has shown that Shh can promote angiogenic blood vessel growth in part by inducing the expression of VEGF as well as Angiopoietin-1 and -2.37) Embryos lacking Shh activity fail to express VEGF within their somites, and exogenous addition of VEGF in these embryos can rescue vascular Ephrin-B2 expression.38)
The Notch signaling pathway is important for cell-cell communication and controls multiple cell differentiation processes during embryonic and adult life. There are four heterodimeric, transmembrane receptors Notch-1 to Notch-4 that are mediators of cell fate.7) Receptor binding of a ligand promotes two proteolytic processing events; as a result of proteolysis, the intracellular domain is freed to enter the nucleus and engage other DNA-binding proteins and regulate gene expression. The four receptors bind to one of five different ligands, Jagged 1-2 or Dll 1-3, the former containing an extra cysteine-rich domain in the extracellular region.37) Because most ligands are also transmembrane proteins, the receptor is normally only triggered from direct cell-to-cell contact.
In the absence of Notch signaling vascular malformations are produced; for example, a loss of Notch signaling in zebrafish leads to a poorly formed dorsal aorta and posterior cardinal vein and arteriovenous malformations.38, 39) Notch signaling is linked to VEGF signaling. For example, by suppressing VEGF activity with antisense morpholino oligonucleotides, arterial marker expression from the dorsal aorta was lost while ectopic arterial expression of venous markers was gained.38)
Of note, Notch signaling has also been associated with aging. For example, in adult skeletal muscle, dedicated stem cells (satellite cells) are responsible for regeneration in response to injury. Satellite cell activation and cell fate determination are controlled by the Notch signaling pathway that is initiated by the rapid increase in expression of the Notch ligand, Delta, following injury.40) Current investigation regarding the role of Notch signaling in aged vasculature may lead to mechanisms for differential responses of young and aged cells to vascular injury.
Gridlock (grl) is downstream of the Notch pathway and is expressed spatially in the lateral posterior mesoderm; it is an artery-specific gene.39) Zhong et al. demonstrated in zebrafish that grl signals the formation of the first embryonic artery. This is well seen in the lateral posterior mesoderm of the zebrafish as the angioblast precursors for the midline trunk artery and vein are distinct as soon as they are formed: both express Fli-1 and flk, but grl is only expressed in the artery where as flt4 is expressed only in the vein.39, 41) Progeny of each angioblast, however, are restricted to only one of the vessels; this arterial-venous fate decision is guided by grl. Moreover, a reduction of grl expression in the zebrafish results in artery ablation with increased Eph-B4 and decreased Ephrin-B2.
Vein Graft Adaptation
This review has demonstrated that the distinction between arterial and venous endothelium is genetically predetermined. Veins are the most commonly used conduit for coronary and peripheral artery bypass; placement of a vein into the higher pressure and flow of the arterial circulation results in adaptation of the vein to the arterial environment, a process termed vein graft “arterialization” or “adaptation.”42, 43) Multiple studies have investigated the pathological and molecular changes in these adapted vein grafts.
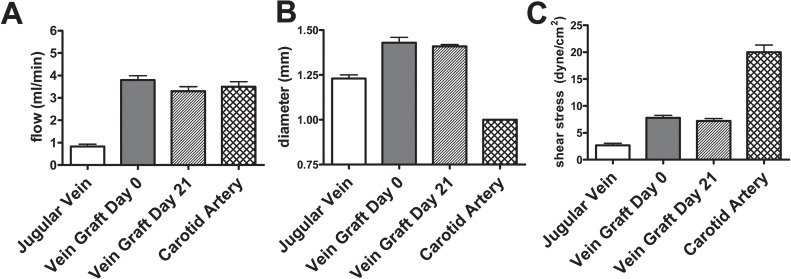
Vein graft “arterialization” involves thickening, fibrosis and subendothelial myointimal cell proliferation. Vein graft thickening is thought to be a response that attempts to reduce the increased magnitude of shear stress to which venous endothelium is exposed upon placement into the higher flow of the arterial circulation, “normalizing” the shear stress down to lower “venous” magnitudes (Fig. 3). Some of the signal transduction pathways that are rapidly stimulated in the venous endothelium upon exposure to the increased pressure and shear stress in the arterial environment include stimulation of the VEGF and PDGF growth factors, and activation of the mitogen-activated protein kinase and the phosphatidyl inositol-3-kinase-Akt signal transduction pathways.44, 45) These factors and pathways lead to increased gene transcription.46, 47)
Fig. 3.
Increased flow through a vein graft (A) leads to graft dilation (B), reducing shear stress (C) within the vein graft.
Using patent saphenous vein grafts explanted from human patients undergoing cardiac transplant, or from patients with peripheral bypass undergoing amputation for distal ischemia without infection or a thrombosed vein graft, Kudo et al.48) demonstrated loss of Eph-B4 expression and protein, without increased expression of Ephrin-B2. These results were suggestive of a loss of venous identity without a gain of arterial identity. Similarly, in a rat model, the intima-media thickening during vein graft adaptation to the arterial environment was associated with short-term loss of the venous phenotype marker Eph-B4, but without expression of the arterial phenotype marker Ephrin-B2 (Fig. 4); interestingly, Eph-B4 was detectable in the venous smooth muscle cells48) (Fig. 4). These results show that aged rats and humans have markers of vessel identity that are plastic during adult life; they suggest that loss of venous identity, rather than gain of arterial identity, is a crucial mechanism for vein graft adaptation, although longer term studies regarding the identity of vein grafts are still needed.48)
Fig. 4.
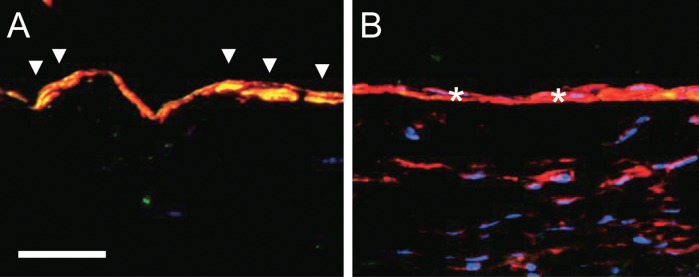
Vein graft adaptation in rats. Photomicrographs of jugular vein (A) and vein graft explanted at 21 days (B) in rats, processed for immunofluorescence to detect Eph-B4 (green), the smooth muscle cell marker α-actin (red), and the nuclear marker DAPI (blue). Scale bar, 20 microns. Arrowheads show colocalization of Eph-B4 with α-actin in the vein, consistent with the presence of Eph-B4 in venous smooth muscle cells; stars show loss of colocalization and Eph-B4 in the vein graft, with persistent detection of the smooth muscle cells.
Unbalanced Molecular Markers Are Abnormal
While vein graft adaptation results in a loss of venous identity, induction of arterial markers may be pathogenic. Lugli et al. described strong Eph-B2 positivity in tumors of the lung and pancreas without Eph-B2 positivity in the corresponding normal cells.49) Similar findings were discovered in colon carcinoma tissue and their adjacent cells.50) Hainaud et al. demonstrated that a coordinated activation of Dll4/Notch-4 and Ephrin-B2 pathways downstream of VEGF plays a key role in the abnormal remodeling of tumor vessels. Also, inhibition of VEGF tends to normalize tumor vasculature and can potentially be used in antiangiogenic therapy.51)
The highly vascular nature of Kaposi's sarcoma and its likely endothelial origin prompted investigation of Eph-B4 and Ephrin-B2 expression in these lesions.52) The normal artery-vein demarcation seen in a capillary bed is altered in the Kaposi's sarcoma lesion as there is expression of Ephrin-B2 but not Eph-B4 on these tumor cells.52) These results suggest that abnormal regulation of Ephrins and/or Ephs may be involved in tumorigenesis. Interestingly, knock down of Ephrin-B2 with siRNA reduced Kaposi's sarcoma cell viability.52)
Arteries and veins are structurally and molecularly distinct. Extensive research has identified distinctive arterial and venous markers that are required for vascular development and physiology. It is important to differentiate artery- and vein- specific receptor-ligand interactions to help understand vascular pathology and processes such as vein graft adaptation. The molecular fingerprints of vasculature are predetermined but plastic. Further exploration regarding the regulation of these markers could help identify additional targets for treatment.
References
- Cavalieri S, Rotoli M. [Huangdi Neijing: a classic book of traditional Chinese medicine]. Recenti Prog Med. 1997; 88: 541–6 [PubMed] [Google Scholar]
- Sakula A. Kaitai Shinsho: the historic Japanese translation of a Dutch anatomical text. J R Soc Med. 1985; 78: 582–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Daya SK, Gowda RM. Evolution of the theory of circulation. Int J Cardiol. 2005; 98: 519–21 [DOI] [PubMed] [Google Scholar]
- Silverman ME. William Harvey and the discovery of the circulation of blood. Clin Cardiol. 1985; 8: 244–246 [DOI] [PubMed] [Google Scholar]
- Zareba KM. Circulation over the centuries: William Harvey (1578–1657). Cardiology Journal. 2007; 14: 214–5 [PubMed] [Google Scholar]
- Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med. 2004; 14: 308–13 [DOI] [PubMed] [Google Scholar]
- Karsan A. The role of notch in modeling and maintaining the vasculature. Can J Physiol Pharmacol. 2005; 83: 14–23 [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. San Diego: Academic Press, 1999 [Google Scholar]
- Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res. 2002; 11: 5–7 [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001; 128: 3359–70 [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001; 49: 507–21 [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000; 6: 389–95 [DOI] [PubMed] [Google Scholar]
- Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003; 23: 1143–51 [DOI] [PubMed] [Google Scholar]
- Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007; 8: 35–42 [DOI] [PubMed] [Google Scholar]
- Gray H.Gray's Anatomy: Anatomy, Descriptive, and Surgical 1901 Edition. In: Hawden R. ed. 1901 [Google Scholar]
- Ross MH, Kaye GI, Pawlina W. Histology Text and Atlas. Lippincott Williams & Wilkins, 2003; 4th Edition. [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998; 93: 741–53 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001; 128: 3675–83 [DOI] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004; 18: 901–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005; 435: 98–104 [DOI] [PubMed] [Google Scholar]
- Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007; 212: 237–48 [DOI] [PubMed] [Google Scholar]
- Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999; 277(3 Pt 2): H1167–77 [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002; 3: 475–86 [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998; 21: 309–45 [DOI] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. Eph receptors, ephrins, and synaptic function. Neuroscientist. 2004; 10: 304–14 [DOI] [PubMed] [Google Scholar]
- Bennett BD, Zeigler FC, Gu Q, Fendly B, Goddard AD, Gillett N, et al. Molecular cloning of a ligand for the EPH-related receptor protein-tyrosine kinase Htk. Proc Natl Acad Sci U S A. 1995; 92: 1866–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004; 7: 501–9 [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004; 16: 580–9 [DOI] [PubMed] [Google Scholar]
- Torres-Väzquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell Tissue Res. 2003; 314: 43–59 [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996; 17: 9–19 [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999; 13: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Bruckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R. Similarities and differences in the way transmembrane-type ligands interact with the Elk subclass of Eph receptors. Mol Cell Neurosci. 1996; 8: 199–209 [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999; 4: 403–14 [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999; 56: 794–814 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999; 5: 1359–64 [DOI] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ. Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res. 1999; 253: 25–33 [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001; 7: 706–11 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002; 3: 127–36 [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001; 414: 216–20 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005; 4: 407–10 [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998; 197: 248–69 [DOI] [PubMed] [Google Scholar]
- Henderson VJ, Cohen RG, Mitchell RS, Kosek JC, Miller DC. Biochemical (functional) adaptation of “arterialized” vein grafts. Ann Surg. 1986; 203: 339–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush HL, Jr, Jakubowski JA, Curl GR, Deykin D, Nabseth DC. The natural history of endothelial structure and function in arterialized vein grafts. J Vasc Surg. 1986; 3: 204–15 [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovascular Res. 2001; 49: 568–81 [DOI] [PubMed] [Google Scholar]
- Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE. 2006; 2006: pe39. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardeña G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001; 98: 4478–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles D, Kwei S, Stavrakis G, Zhang Y, Wang ET, Garcia-Cardeña G. Gene expression changes evoked in a venous segment exposed to arterial flow. J Vasc Surg. 2006; 44: 863–70 [DOI] [PubMed] [Google Scholar]
- Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007; 27: 1562–71 [DOI] [PubMed] [Google Scholar]
- Lugli A, Spichtin H, Maurer R, Mirlacher M, Kiefer J, Huusko P, et al. EphB2 expression across 138 human tumor types in a tissue microarray: high levels of expression in gastrointestinal cancers. Clin Cancer Res. 2005; 11: 6450–8 [DOI] [PubMed] [Google Scholar]
- Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan F, Bucana CD, et al. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer. 2002; 94: 934–9 [DOI] [PubMed] [Google Scholar]
- Hainaud P, Contrerès JO, Villemain A, Liu LX, Plouët J, Tobelem G, et al. The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res. 2006; 66: 8501–10 [DOI] [PubMed] [Google Scholar]
- Masood R, Xia G, Smith DL, Scalia P, Still JG, Tulpule A, et al. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arterial endothelium. Blood. 2005; 105: 1310–8 [DOI] [PubMed] [Google Scholar]