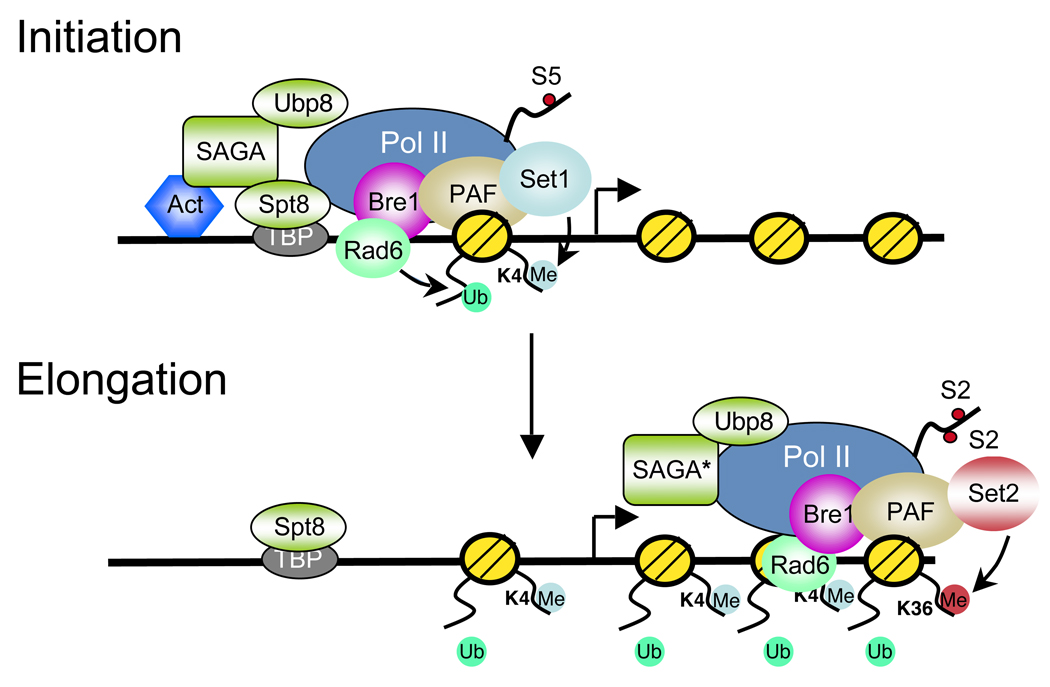
Figure 1. Dynamic ubiquitylation and deubiquitylation of H2B during transcription initiation and elongation.
Activators such as Gal4 recruit the Bre1/Rad6 and SAGA histone acetyltransferase/deubiquitinase complexes to gene promoters. RNA polymerase II (Pol II) CTD phosphorylation on serine 5 (S5) by Kin28 recruits the PAF complex, which in turn recruits Rad6/Bre1 and the Set1 histone methytransferase complex to Pol II. This association results in H2BK123ub1 and H3K4me3 formation at the promoter and 5’ coding region during the initiation phase of transcription. H2BK123ub1 is a prerequisite for Set1-mediated H3K4me3 in these regions. A SAGA subcomplex (*) that contains the Ubp8 H2B ubiquitin protease module also associates with Pol II and deubiquitylates H2BK123ub1. Both the Bre1/Rad6 and the SAGA-Ubp8 complexes travel with Pol II across the coding region and lead to a cycle of H2B ubiquitylation and deubiquitylation during transcription elongation. The dynamic regulation of H2B ubiquitylation has at least two functions during this phase of transcription. First, deubiquitylation of H2B by Ubp8 allows Ctk1 recruitment and Pol II CTD phosphorylation on serine 2(S2). This in turn results in the recruitment of the Set2 histone methyltransferase and the formation of H3K36me3 at the 3’ coding region. Second, H2B ubiquitylation plays a role in nucleosome dynamics by promoting nucleosome reassembly in the wake of elongating Pol II. Both functions are postulated to contribute to the fidelity of transcription elongation.