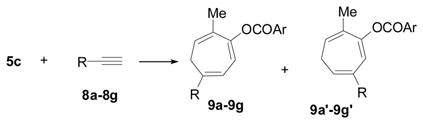
Table 2.
Rh-Catalyzed [5 + 2] Cycloaddition of 5c and Alkynes (Ar = p-Me2NC6H4)a
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Alkyne Substrates | Yield | Ratio of (9: 9′) | ||||
| 1 | 7 | 6c, 97% | only 6c | ||||
| 2 |
8a,

|
9a, 87% | only 9a | ||||
| 3 |
8b,

|
9b, 89% | >20:1 | ||||
| 4 |
8c,
|
9c, 95% | only 9c | ||||
| 5 |
8d,
|
9d, 96% | 17:1 | ||||
| 6 |
8e,
|
9e, 74% | only 9e | ||||
| 7b |
8f,

|
9f, 58% | >20:1 | ||||
| 8c |
8g,

|
9g, 77% | 8:1 | ||||
| 9 |
8h,
|
9h, 82% | - | ||||
| 10c |
8i,
|
9i, 89% | - | ||||
| 11b |
8j,
|
9j, 77% | 5:1 | ||||
| 12 |
8k,
|
complex mixture | |||||
| 13 |
8l,
|
complex mixture | |||||
Conditions: [Rh(PPh3)3Cl] (0.5 mol %), alkyne 7 or 8 (1.1 equiv), CHCl3 (0.4 M), 50 °C, 24h unless noted otherwise. Isomeric ratios were determined by 1H NMR of crude products.
[Rh(PPh3)3Cl] (2 mol %)
[Rh(PPh3)3Cl] (10 mol %)