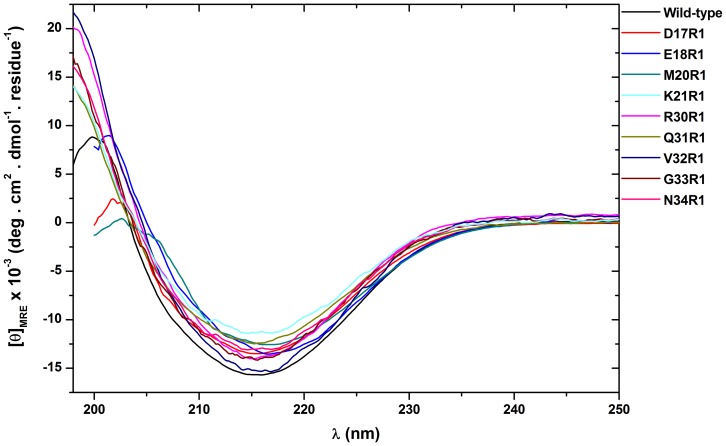
Figure 2. CD spectra of B-FABP wild type and their mutants in 20 mM phosphate buffer (pH 8.0).
All spectra are characterized by a negative minimum around 215 nm, which is typical in β-rich proteins. The similarity of the spectra suggests that the introduction of the Cys residue in the mutants of B-FABP has not significantly altered their overall secondary structure arrangement.