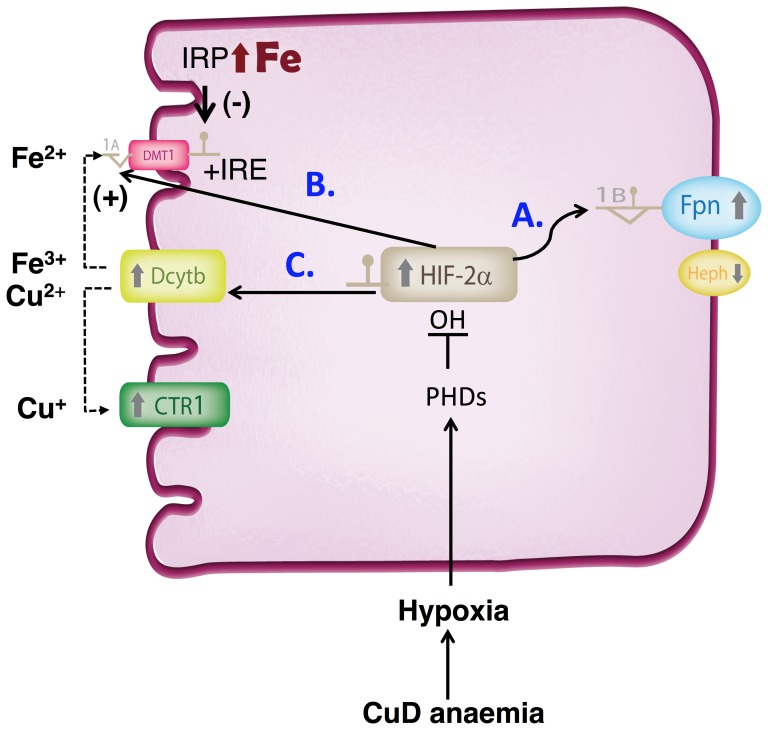
Figure 5. Model for the role of HIF-2α in the adaptive response to copper deficiency anemia.
HIF-2α upregulation results in altered expression of iron absorption genes as a result of systemic copper deficit/anemia. This leads to: a) increases in Fpn levels to compensate/adjust the rates of iron export to systemic copper decreases; b) systemic modulation of DMT1+IRE expression (HIF-2α trans-activation of the DMT1-1a promoter). The net expression of DMT1 is likely influenced by the amount of iron retained in the gut (associated with CuD due to a significant decrease in hephaestin feroxidase activity), and the effect on IRP activity. On one hand, in mild or moderate CuD (no iron retention) systemic HIF-2α increases are likely to exert an overall dominant effect on DMT1 expression (Figure 2). At the other extreme, in severe CuD resulting in enterocyte iron loading, decreases in IRP stability and thus Dmt1+IRE mRNA half-life, would counterbalance the HIF-2α transcriptional upregulation of DMT1, thus decreasing, or not altering DMT1 expression (Figure S2); c) increases of Dcytb in CuD, apart from iron uptake and depending on context, could also promote copper uptake in tandem with Ctr1.