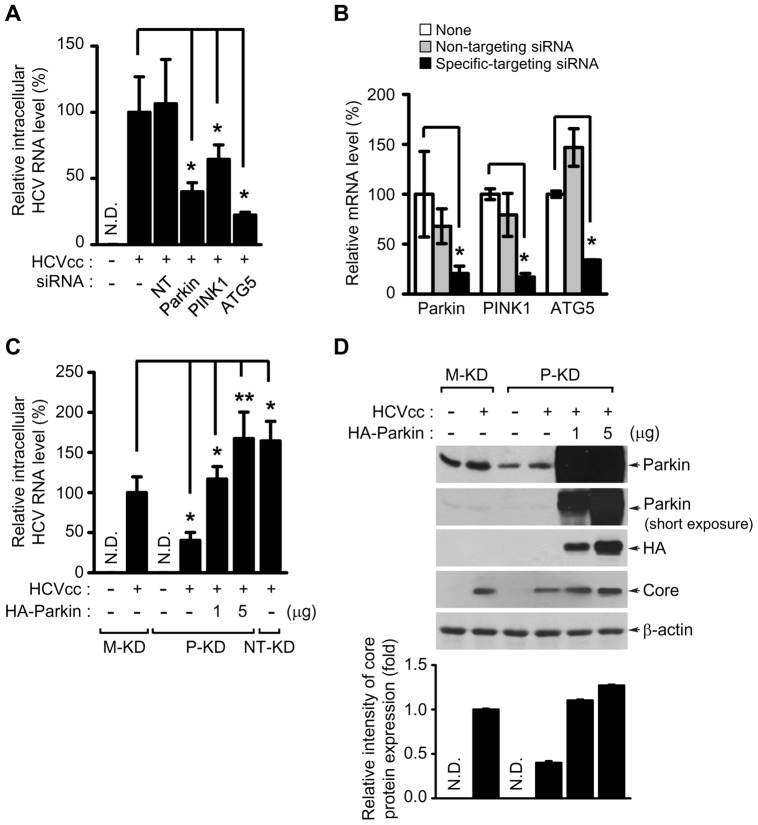
Figure 6. Parkin and PINK1 affects HCV replication.
(A and B) Inhibitory effect of Parkin, PINKI, and ATG5 silencing on HCV replication. Huh7 cells transfected with Non-Targeting (NT) or gene-specific siRNA pools targeting Parkin, PINK1, and ATG5 genes, respectively, were infected with HCVcc. At day 3 post-infection, the levels of HCV RNA (A) and targeted gene mRNA (B) were analyzed by real-time qRT-PCR, as described in Materials and Methods (mean ± SD; n = 3; *p<0.01). GAPDH was used to normalize changes in Parkin, PINK1, and ATG5 gene expression. (C and D) Rescue of HCV RNA replication by Parkin overexpression. Huh7 cells stably expressing mock vector (M-KD), Non-target-shRNA (NT-KD), and Parkin-shRNA (P-KD), respectively, were infected with HCVcc. P-KD cells were further transfected with two different concentration of the plasmid DNA encoding wild-type Parkin for 2 days before harvest. At 3 days post-infection, intracellular HCV RNA levels were analyzed by real-time qRT-PCR. GAPDH was used to normalize changes in HCV RNA expression (C) (mean ± SD; n = 3; *p<0.01, **p<0.05). (D) The expression levels of Parkin and HCV core protein were analyzed by immunoblotting with anti-Parkin and HCV core antibodies. Ectopic expression level of HA-tagged wild-type Parkin was detected by immunoblotting with anti-HA antibody. β-actin was used as an internal loading control. P values were calculated by using an unpaired Student's t-test. The relative intensity of HCV core expression normalized to β-actin was analyzed by ImageJ.