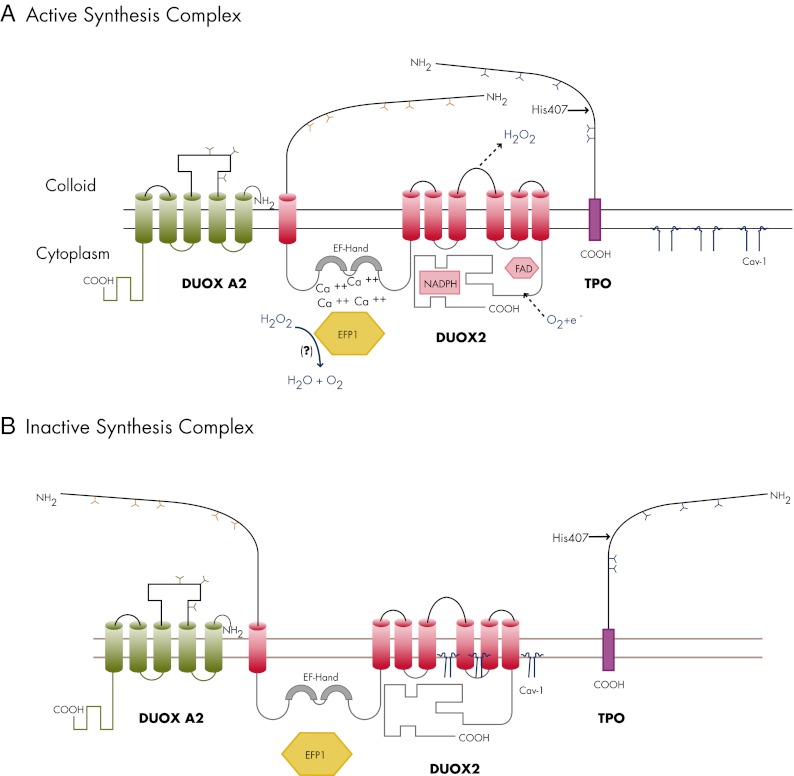
Figure 3.
The multiprotein complex involved in TH biosynthesis. A, Upon TSH stimulation, DUOX2 and TPO migrate into the plasma membrane where the complex is activated. TPO and DUOX2 are viewed as a producer-consumer unit that allows TPO to use H2O2 instantly to oxidize iodide (I−) into iodine, to iodinate tyrosyl residues of Tg, and to couple iodotyrosyl residues to form T3 and T4, while protecting the cell against H2O2 possible oxidative damage. DUOX2 is represented here as a seven-transmembrane-domain glycoprotein with an extracellular NH2-terminal peroxidase-like domain that appears essential for interaction with TPO, a long COOH-terminal region that contains the catalytic NADPH oxidase core along with the flavin adenine dinucleotide (FAD) and NADPH binding cavities, and two EF-hands in the first intracellular loop that are essential calcium binding sites. TPO is represented with a short intracellular COOH terminus and a long extracellular NH2 terminus (90% of its amino acids) that exhibits a catalase-like activity in certain circumstances. His407 is involved in the covalent binding of the heme prosthetic group that is essential for enzyme activity. The manner in which TPO and DUOX2 interact makes the apical multiprotein complex perfectly suited to detoxify ROS and avoid H2O2 spillages. The local H2O2 concentration is kept under control by the TPO catalase-like action (which protects DUOX2, as long as both proteins are closely associated). In addition, the complex contains the thioredoxin-related protein EFP1, which interacts with the two EF-hands of DUOX2 and detoxifies H2O2 that is not readily used in the biosynthesis process. EFP1 may also be involved in the maturation process of DUOX2. For this biochemical unit to be activated, DUOX2 must be fully glycosylated (Y, N-glycosylation sites) following successive maturation steps in the ER and the Golgi apparatus. DUOX2 must also be associated with the maturation factor DUOXA2, which is represented here as a five-transmembrane-domain protein with an extracellular loop between the second and the third transmembrane domains along with three glycosylation sites (Y). I− and H2O2 regulate the activity of the enzymatic complex, depending on their local relative concentration. When I− is present in adequate amounts, H2O2 is the limiting factor of the reaction and mediates the association between TPO and DUOX2. In thyroids with low intracellular I− content, I− instead exerts a stimulatory effect on H2O2 production. When I− is present in excess, H2O2 synthesis is blocked. Because I−-induced effects are inhibited by methimazole or propylthiouracil, this indicates that I− acts through oxidized species. B, In resting conditions, the complex TPO-DUOX2-DUOXA2 is kept inactive underneath the apical membrane. Cav-1 is required to keep DUOX2 quiescent, because it may interact with domains in the cytoplasmic region of the molecule that are putative sites for Cav-1 scaffolding domains (following a hypothesis that still needs to be proved). According to the most plausible explanation, Cav-1 would come off the enzyme complex after its incorporation into the apical membrane. The absence of Cav-1 is responsible for mislocalization and premature activation of the complex in the cytoplasm, creating potentially devastating consequences due to increased OS and/or lack of efficient antioxidant mechanisms naturally activated when I− organification and coupling reactions normally occur in apical microvilli.