Abstract
Humans and rodents show large variability in their individual sensitivity to diet-induced obesity, which has been associated with differences in intrinsic spontaneous physical activity (SPA). Evidence from genetic and out-bred rat obesity models shows that higher activity of the orexin peptides results in higher intrinsic SPA and protection against diet-induced obesity. Based on this, we hypothesized that naturally occurring variation in SPA and orexin signaling activity is sufficient to drive differences in sensitivity to diet-induced obesity. We analyzed orexin activity and sensitivity to diet-induced obesity in non-manipulated male Sprague Dawley rats selected for high and low intrinsic SPA. Our results defined a new model of differential DIO sensitivity, the high-activity and low activity-rats, and suggest that naturally occurring variations in intrinsic SPA cause differences in energy expenditure that are mediated by orexin signaling and alter DIO sensitivity.
Keywords: Orexin, hypocretin, hypothalamus, obesity, diet-induced obesity, high-fat diet, spontaneous physical activity
Introduction
Humans and rodents show large variability in their individual sensitivity to diet-induced obesity (DIO) (1–6) and lower DIO sensitivity has been correlated with higher levels of intrinsic physical activity (SPAINT) (7–9). SPAINT is defined as “physical activity that does not qualify as voluntary exercise” (10, 11). In rodents, SPAINT is measured as spontaneous ambulatory movement plus rearing in an open field over a long period of time (i.e., 24 h) after adaptation to the new environment to avoid a confound effect of initial exploratory activity (11–14). Currently, the neurobiological control of SPAINT is understood as a distributed brain network involving multiple neuropeptide systems, which includes the orexins/hypocretins (10, 15). There are two orexin peptides (orexin-A/hypocretin-1, OXA, and orexin-B/hypocretin-2, OXB) and two receptors (orexin/hypocretin receptor 1, OX1R, and orexin/hypocretin receptor 2, OX2R) (16, 17). The orexin neurons are located in the lateral hypothalamic and perifornical areas and have efferents to multiple brain sites (18, 19) with varying orexin receptors (OXR) expression levels (20–23).
Activation of the orexin receptors promotes negative energy balance, probably through an increase and/or maintenance of SPAINT. This view of orexin’s function is supported by multiple lines of evidence: mice deficient in orexin neurons show lower levels of physical activity, hypophagia and obesity (24); over-expression of the orexin peptides leads to resistance against diet-induced obesity (25); injection of orexin peptides in several brain sites increases SPA (12, 13, 26) and can cause weight loss (27); finally, systemic injection of orexin receptor antagonists decreases SPA (27). These data show that higher orexin signaling leads to higher SPAINT resulting in resistance to obesity. Our previous work in the obesity prone (OP) and obesity resistant (OR) out-bred rats (28) supports this hypothesis, as the OR phenotype shows higher SPAINT levels, higher orexin behavioral responsivity (14) and long-term DIO resistance (9). However, the OP and OR rats are not bred for differences in SPA but for weight gain under an obesogenic diet (28). Thus, it is possible that higher SPAINT in OR rats is a trait that cosegregates with DIO resistance. Therefore, the OP/OR model does not conclusively prove that differences in SPAINT are sufficient to drive differential DIO sensitivity. Together, these data suggest the variation in SPAINT in non-bred rodent populations and the potential to impact DIO sensitivity remains unknown.
The current studies were designed to test the following hypothesis: Natural (i.e., un-manipulated) differences in SPAINT are sufficient to drive differences in DIO sensitivity. Furthermore, we hypothesized that orexin function might underlie the variation in SPAINT. In these studies, we selected male Sprague Dawley rats for differences in ambulatory SPAINT levels and tested their energy expenditure, prepro-orexin mRNA expression; behavioral effects of orexin and DIO sensitivity. Our work defined a new model of differential DIO sensitivity: high-activity and low activity-rats. These results suggest that naturally occurring variations in SPAINT are related to differences in activity of the orexin peptides and are sufficient to drive differences in energy expenditure and protect against diet-induced obesity.
Methods
Animals
Male Sprague-Dawley (SD) rats (Charles River, Kingston, NY, USA; 200–250µg at arrival) were individually housed in hanging-wire cages with a 12-h light/dark cycle (lights on at 06:30 AM) 21–22°C. Animals were given one week of acclimation to housing conditions with food and water ad libitum. The experiments were approved by the Local Institutional Animal Care and Use Committee at the Minneapolis VA Medical Center.
Diets
High fat (HF, D12451; 45% kcal from fat;) and low fat (LF, D12450B; 10% kcal from fat) from Research Diets (New Brunswick, NJ, USA) and standard diet (Harlan Teklad 8604) were used. Food and water were available ad libitum.
Peptides
Orexin-A (American Peptides, Sunnyvale, CA, USA) was dissolved in artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Holliston, MA, USA), aliquoted and kept at −20°C until needed.
Body weight, fat and lean mass and food intake measurements
Body weight (g) and food intake corrected by spillage (g) were determined every other day. Total fat mass (g) and lean mass (g) were measured by quantitative magnetic resonance (29, 30) using the EchoMRI-900 scan (Echo Medical Systems, Houston, TX, USA).
Measurement of SPAINT
SPA was measured in a 17.0 by 17.0 inches squared acrylic cage surrounded by three 16-beams sets of infrared activity sensors (Med Associates, St. Albans, VT, USA). Two sets of arrays were in the x-y plane and the third set was elevated 3 inches above the x-y plane. Movement was recorded by beam breaks with 100 ms resolution. Intrinsic SPA levels were measured over a 24 h period after 24 h habituation to the recording cages. This length of habituation time is sufficient to produce stable SPAINT measurements (Supplementary Figure 1). Ambulatory intrinsic SPA (SPAINT) levels are reported in min as time spent moving lateral plus time spent rearing. Stereotypic SPAINT is defined as time spent performing any partial-body movements (i.e. grooming) within a defined space around the animal (3.25 × 3.25 inches). Measurement of ambulatory and stereotypic SPAINT for classification of rats as HA or LA was the first test conducted in all animals after acclimation to the housing facilities.
Indirect Calorimetry (IDC)
IDC and SPA were recorded simultaneously in an air-tight SPA cage of the same dimensions and configuration as described previously. Oxygen consumption and carbon dioxide production were measured by using a customized, high-precision, single-chamber indirect calorimeter (Columbus Instruments, Columbus, OH, USA). SPA was recorded as described in the previous section. Thermogenesis was calculated from oxygen consumption and carbon dioxide production and is reported as the average kcal per h over 24 h. During IDC recordings, animals had food and water available ad libitum. Rats (N = 32) were acclimated for 7 days prior to the beginning of IDC recordings in the IDC SPA cages. IDC was recorded for 3 consecutive 24 h periods.
Response to OXA in rostral lateral hypothalamus (rLH) and substantia nigra pars compacta (SN)
Rats were prepared with unilateral cannulae targeting rLH (stereotaxic coordinates: −2.0 mm lateral, −2.1 mm posterior to Bregma, 7.3 mm below the skull surface) (31) or SN (stereotaxic coordinates: −2.4 mm lateral, −5.3 mm posterior to Bregma, 7.6 mm below the skull surface) (31). There was one week of recovery from surgery before proceeding with experiments.
Measurement of SPA after orexin-A injection was conducted as follows. During each recording session, animals were transported from their home cages to the SPA recording cages and habituated for 1–2 h. Analysis of the time course of response showed this length of habituation was sufficient to produce a stable baseline in both high and low activity rats (Supplementary Figures 2–3). Next, animals were injected unilaterally with either aCSF or OXA (0.5 µl injected over 30 seconds). After each injection, the injector was left inside the cannula for 30 seconds to ensure diffusion of the injectate. Next, SPA was recorded for 3 h and animals returned to their home cages. All injections were done between 8:00 AM and 11:00 AM.
The injection schedule was as follows. From days 1–3, all rats received injections of aCSF in the SPA cages for acclimation to the cages and injection procedure. Next, rats received a unilateral injection of aCSF or OXA (50, 125, 250 and 500) in a latin-square design to avoid a treatment order confound. At least 48 h elapsed between injections.
Time course analysis showed that SPA during the first 5 min after injection related to the injection procedure itself and were excluded from the data analysis. SPA is reported as time spent moving for 2 h starting 5 min after injection. After completion of the experiment, accuracy of cannula placement was confirmed by histological methods (13).
Real Time PCR analysis
HA and LA rats were euthanized by decapitation between 10:00 and 12:00 PM. Food was removed 2 h before euthanasia. Samples from caudal lateral hypothalamus (cLH) were collected as described previously (32), immediately frozen in liquid nitrogen and stored at −80°C.
Total RNA was extracted using TRIzol (15596-026, Invitrogen, Carlsbad, CA, USA), cleaned using the RNAeasy micro kit (74034, Qiagen, Germantown, MD, USA), quantified by UV absorption at 260 nm with the ND-1000 spectrophotometer (ThermoScientific, Wilmington, DE, USA) and stored at −80°C until use. Equal amounts of RNA were used to measure expression levels of morexin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA with the LightCycler RNA Master SYBR Green I kit (03064760001, Roche, Indianapolis, IN, USA) in a LightCycler 2 thermocycler (Roche). Primers sequences were described previously (14). Efficiency of each PCR reaction was determined using the dilution method (33). Expression levels of prepro-orexin mRNA were normalized against GAPDH using an efficiency corrected formula (33).
Statistical Analysis
All statistical analyses were done with the R software version 2.11.1 (34). Data are presented as mean ± standard error of the mean. Effects of DIO on HA and LA rats were analyzed with a 2-way ANOVA with phenotype (HA/LA) and diet (HF/LF) as independent variables. Analysis of SPA after OXA injections was done using a 2-way repeated measures ANOVA with dose of orexin as the repeat measure and HA/LA phenotype as the independent variable. Feeding efficiency was calculated as kcal necessary for gain of fat or lean mass (i.e. fat mass divided by cumulative kcal consumed) and analyzed with a 2-way ANCOVA analysis using body weight pre-feeding as a covariate and diet (HF/LF) and phenotype (HA/LA) as independent variables. Pairwise comparison of SPAINT levels between HA and LA rats were done with Mann-Whitney-Wilcoxon test as distributions were not normal. Unless indicated otherwise, pair-wise comparisons were done by multiple t-test with pooled variance corrected for multiple comparisons with the method of Hochberg (35).
Analysis of reliability of HA/LA classification was performed on data from 29 male SD for which SPAINT levels were measured twice over the course of 2 weeks. Reliability was calculated as number of HA/LA rats that were classified in the same category based on different measurements. As the SPAINT from HA and LA populations do not follow a normal distribution, bias confidence intervals for the mean and median were calculated by bootstrap analysis.
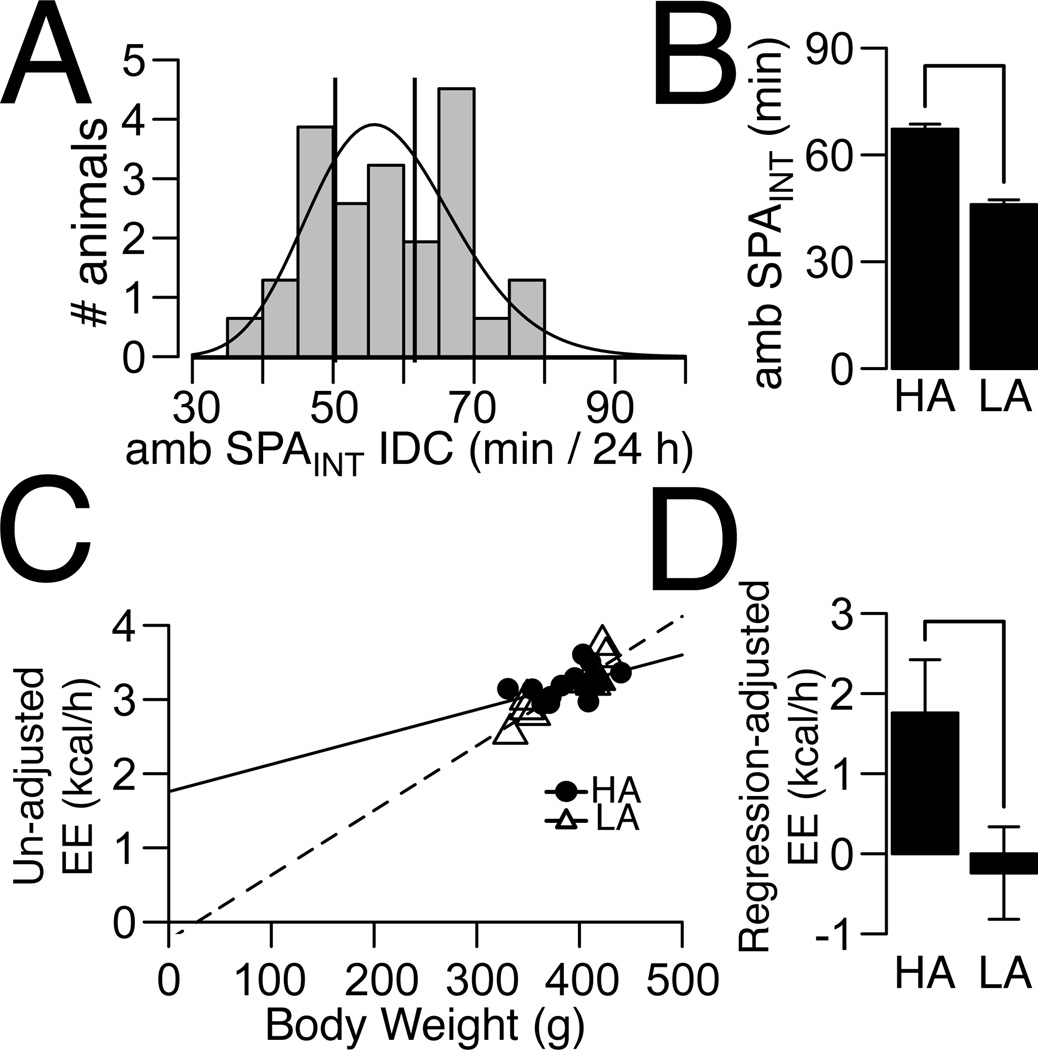
During the first day of IDC recording we observed a reduction in the range of 24 h ambulatory SPAINT (Figure 3A) compared to SPAINT recorded in non-IDC SPA cages (Figure 1A). Therefore, we classified HA and LA rats using the same quantiles from the ambulatory SPAINT distribution recorded in the IDC SPA cages as used in all other experiments (25% quantile for LA and 63% for HA rats respectively). SPA and energy expenditure (EE) were recorded for a total of three 24 h periods. EE was corrected with a linear regression analysis over total body weight independently for HA and LA rats (36, 37) using the averages of uncorrected energy expenditure (kcal/h) and body weight (g) over three consecutive 24 h periods of recording. At the end of the IDC recordings, we measured fat and lean mass in a subset of rats and found no significant differences between HA and LA rats in these measures (Supplementary Table 3), justifying the use of total body weight for correction of energy expenditure. For HA/LA classification, reliability of classification was calculated as before, in a sample of 32 male SD rats; rats were classified as HA/LA based on ambulatory SPAINT measured consecutively over three 24 h periods.
Figure 3. Energy Expenditure (EE) in HA and LA rats.
(A) Distribution of ambulatory intrinsic SPA of Sprague-Dawley rats (N = 32) in the IDC chambers measured for 24 h. EE and SPAINT were recorded for three consecutive 24 h periods, but HA and LA rats were classified using SPA from the first 24 h. Vertical lines indicate cut-off for selection of HA (SPAINT ≥ 61) and LA (SPAINT ≤ 50) corresponding to the 25% and 63% quantile of the SPAINT distribution. (B) Difference in ambulatory SPAINT between HA (N = 14) and LA (N = 10) rats. (C) Linear regression for uncorrected EE over body weight for HA and LA rats. Lines indicate predicted lines from linear regressions. In this analysis the average values of uncorrected EE and body weight over all IDC recording sessions were used. (D) EE corrected by body weight is higher in HA compared to LA rats. Plotted values correspond to intercepts from plot (C). For HA rats, the intercept was significantly different from zero (P = 0.021), but the intercept was not significantly different from zero for LA rats (P = 0.68). Y-axis indicates intercept ± standard error. For plots (B, D), line indicates P ≤ 0.05.
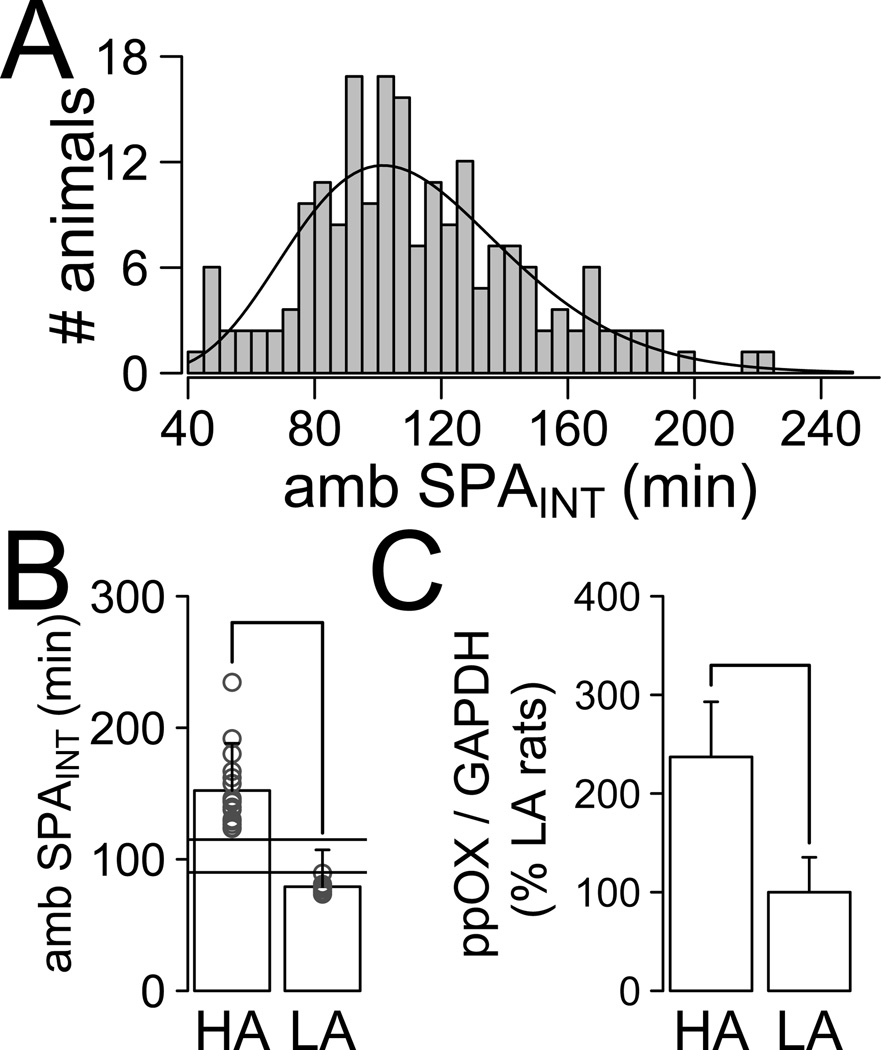
Figure 1. Characterization of the HA and LA phenotype.
(A) Distribution of ambulatory intrinsic SPA (amb SPAINT) measured for 24 h (N = 166). (B) High Activity (HA) and Low Activity (LA) rats used for ppOX have significant differences in ambulatory SPAINT (W = 144, P < 0.001; HA, N = 18, LA, N = 9). Open circles indicate individual animals. (C) Real time PCR analysis showed that HA rats have higher expression of prepro-orexin mRNA (ppOX) in cLH compared to that in LA rats (t23.10 = 2.73, P = 0.011).
For all statistical analysis, a p-value of less than 0.05 was considered significant.
Results
Definition of High-Activity (HA) and Low-Activity (LA) phenotype
We calculated a distribution of 24 h ambulatory SPAINT in male SD rats on a standard diet (Figure 1A, N = 166 male SD rats). This distribution had a positive skewness (0.053) and was described by a gamma distribution (KS test, D = 0.039, P = 0.95). There was a significant correlation of ambulatory SPAINT with lean mass (LM, ρ = 0.172, P = 0.032) and total body weight (ρ = 0.162, P = 0.042) but not with fat mass (FM, ρ = −0.001, P = 0.98) or FM/LM (ρ = −0.075, P = 0.349).
We used the ambulatory SPAINT distribution to define HA (SPAINT ≥ 120 min, 63% quantile of the ambulatory SPAINT distribution) and LA (SPAINT ≤ 90 min, 25% quantile of the ambulatory SPAINT distribution) rats. While this criterion is arbitrary, it results in significant differences in SPAINT between HA and LA rats (Figure 1B), and comprises 88% of all animals in the population. Descriptive statistics of the HA and LA rats are shown in Table 1. Importantly, there were no significant differences in total body weight, fat mass, lean mass or fat to lean mass ratio between HA and LA rats at study onset (Table 1), which did not depend on the criteria for selection of HA or LA rats (Supplementary Tables 1–3). Although we defined the HA/LA phenotype based on ambulatory SPAINT, SD rats also show stereotypic physical activity (See Methods for Definition of ambulatory and stereotypic SPA). A linear correlation analysis showed a small, but statistically significant correlation between ambulatory SPAINT and stereotypic SPAINT (ρ = 0.241, P = 0.002). Likewise, there was a small, but statistical significant difference in stereotypic SPA between HA and LA rats (W = 1609.5, P = 0.014, See Table 1).
Table 1.
Descriptive Statistics for SPAINT Distribution for High and Low Activity Rats
| High Activity | Low Activity | |
|---|---|---|
| Mean Ambulatory SPAINT [BCA 95% CI]a, b | 149.18 [144.0, 155.9] (N = 61) | 71.90 [67.50, 75.75] (N = 41) |
| Median Ambulatory SPAINT [BCA 95% CI]a, b | 143.18 [136.9, 146.7] (N = 61) | 76.22 [69.72, 81.36] (N = 41) |
| Mean Stereotypic SPAINT [BCA 95% CI]a, b | 97.17 [93.80, 101.92] (N = 61) | 90.85 [85.59, 97.87] (N = 41) |
| Median Stereotypic SPAINT [BCA 95% CI]a, b | 94.63 [90.86, 97.81] (N = 61) | 88.15 [78.22, 91.72] (N = 41) |
| Body Weight [Mean ± SEM]c | 291.80 ± 5.20 (N = 56) | 282.25 ± 5.35(N = 39) |
| Fat Mass [Mean ± SEM]c | 29.00 ± 0.58 (N = 56) | 28.49 ± 0.79 (N = 39) |
| Lean Mass [Mean ± SEM]c | 235.22 ± 4.04 (N = 56) | 228.2 ± 3.86 (N = 39) |
| Fat Mass / Lean Mass [Mean ± SEM]c | 0.124 ± 0.0021 (N = 56) | 0.124 ± 0.0024 (N = 56) |
Confidence intervals are from bootstrap calculation using bias correction.
P-values for comparisons between HA and LA < 0.05.
P-values for comparisons between HA and LA were not significant
In a subset of SD rats, we repeated the measure of ambulatory SPAINT one week apart and calculated the reliability of classification of SD rats as HA/LA as 72.85% (See Statistical Methods).
Prepro-orexin mRNA expression and rLH and SN orexin responsivity in HA and LA rats
As genetic manipulation of orexin expression levels directly alters SPAINT (24, 25) we hypothesized that differences in orexin contribute to differences in ambulatory SPAINT. Analysis of expression of the prepro-orexin mRNA (17) in cLH samples from HA (N = 18) and LA (N = 9) rats (Figure 1B) showed that HA rats had higher expression of prepro-orexin mRNA compared to LA rats (Figure 1C, Welch t-test, t = 2.73, df = 23.11, P = 0.012).
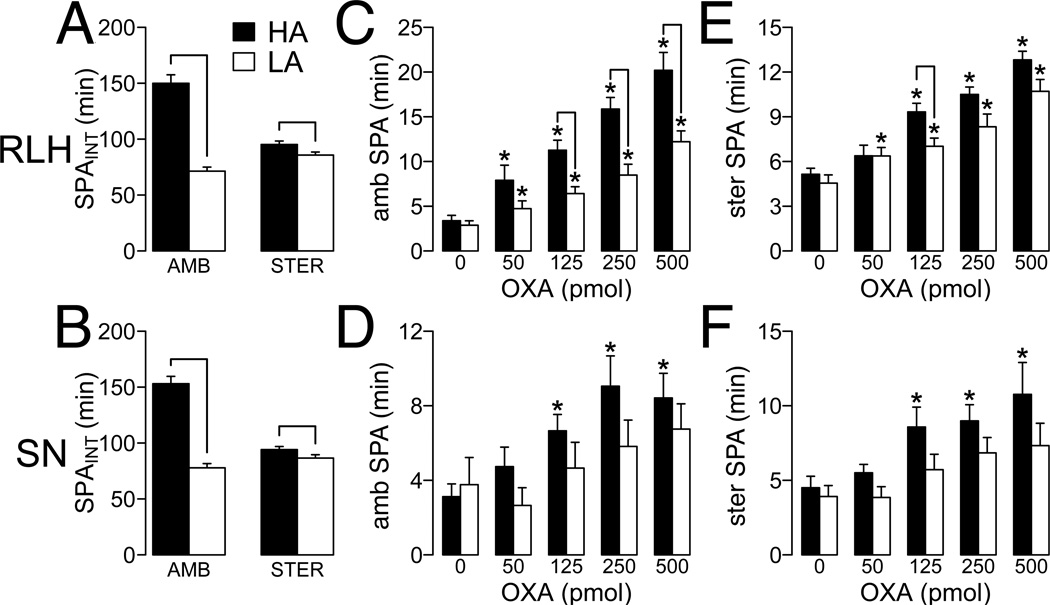
In the OP/OR model, higher levels of ambulatory SPAINT correlate with higher rLH orexin responsivity (14), and orexin regulation of SPA is distributed through multiple brain sites including the rLH and SN (13). Therefore, we hypothesized that HA rats will have higher rLH and SN orexin responsivity compared to LA rats. Figures 2A and 2B show the ambulatory and stereotypic SPA in HA and LA rats for rLH (HA, N = 14; LA, N = 13; Figure 2A) and SN (HA, N = 11; LA, N = 7; Figure 2B) injections. A 2-way ANOVA with dose of OXA as a repeat measure showed that injection of OXA in rLH and SN significantly increased ambulatory SPA (rLH: Figure 2C, F4,100 = 53.42, P < 0.01; SN: Figure 2D, F4,64 = 6.01, P < 0.01). In rLH, comparison of individual doses of OXA against aCSF injection showed significant differences in HA and LA rats across all doses of orexin (Figure 2C). On the contrary, for SN there were only significant differences within HA rats at higher OXA doses (Figure 2D). There was a significant effect of the HA/LA phenotype on ambulatory SPA after OXA rLH injection (F1,25 = 17.17, P < 0.01). In SN, the effect of the HA/LA phenotype approached, but failed to reach statistical significance (F1,19 = 3.39, P = 0.08). Pairwise analyses showed significant differences between HA and LA rats at all doses of OXA in rLH (Figure 2C), but failed to find significant differences in SN (Figure 2D).
Figure 2. SPA after rostral lateral hypothalamus (rLH) and substantia nigra (SN) injection of orexin-A (OXA).
SPA after injection of orexin was measured for 2 h post-injection after 1 h acclimation to the testing chambers. (A) Ambulatory (AMB) and (B) stereotypic (STER) SPAINT in high activity (HA) and low activity (LA) rats used for analysis (for RLH, HA, N = 13; LA, N = 14; for SN, HA, N = 11; LA, N = 7). No significant differences in ambulatory or stereotypic SPAINT were found within HA (ambulatory, W = 99, P = 0.813; stereotypic, W = 105, P = 1.000) or LA rats (ambulatory, W = 52, P = 0.276; stereotypic, W = 68, P = 0.84) selected for orexin injections in rLH or SN. Line: P ≤ 0.05 for the Mann-Whitney-Wilcoxon test. (C,D) Ambulatory SPA after OXA injection in RLH (C) and SN (D). (E, F) Stereotypic SPA after OXA injection in RLH (D) and SN (E). Line: P ≤ 0.05 for pairwise comparison between HA and LA at each OXA dose; *P ≤ 0.05 for pairwise comparison within HA and LA rats at each OXA dose vs aCSF injection. For (C–F) all p-values were corrected for multiple comparisons (See Methods, Statistical Analysis).
Analysis of stereotypic SPA after OXA injection indicated a significant main effect of OXA dose in rLH (Figure 2E, F4,100 = 46.21, P < 0.01) and SN (Figure 2F, F4,64 = 7.27, P < 0.01). In rLH, comparison of individual doses of OXA against aCSF injection showed significant differences in HA and LA rats across doses of orexin (Figure 2E). On the contrary, for SN there were only significant differences within HA rats at higher OXA doses (Figure 2F). As with ambulatory SPA, there was a significant effect of the HA/LA phenotype for rLH OXA injections (F4,100 = 7.023, P = 0.013), whereas this failed to reach statistical significance for SN OXA injections (F416 = 4.02, P = 0.062). Pairwise analysis of stereotypic SPA response to OXA in rLH of HA and LA rats showed significant differences only at one dose of OXA (Figure 2E) while for SN injections, there were no statistical differences between HA and LA rats at individual doses (Figure 2F). Together, these data suggest that differences in rLH and SN orexin responsivity contribute to the differences in ambulatory and stereotypic SPA between HA and LA rats, with the rLH effect being more robust.
Energy Expenditure (EE) in HA/LA rats
We used indirect calorimetry (IDC) to test the hypothesis that HA rats have higher EE than LA rats. The distribution of ambulatory SPAINT over 24 h collected in the IDC cages (Figure 3A) had a smaller range compared to non-IDC SPA cages (Figure 1A), despite using cages of the same size (See Methods). Therefore, we classified HA and LA rats from the distribution of ambulatory SPAINT collected in the IDC cages using the same quantiles used to define HA/LA rats from the distribution shown in Figure 1A. Similar to the original ambulatory SPAINT distribution (Figure 1A), the distribution of ambulatory SPA recorded in the IDC cages had a positive skewness (0.054) and was described by a gamma distribution (KS test, D = 0.017, P = 0.83). Despite the reduced range of ambulatory SPAINT, there were significant differences in ambulatory SPAINT between HA and LA rats (Figure 3B; t22 = 10.57, P < 0.001). Next, we recorded EE and ambulatory SPAINT for two additional 24 h periods. We found no significant differences in body weight or food intake between HA and LA rats across recording sessions (Supplementary Table 2). Therefore, we used the average values of un-corrected EE and body weight over the three 24 h periods of recording to compare EE between HA and LA rats. Importantly, we maintained the classification of HA and LA rats based on ambulatory SPAINT from the first 24 h of recording. Figure 3C shows the best-fit lines for HA and LA rats for un-corrected EE over total body weight averaged over the recording sessions. After adjustment for total body mass HA rats showed significantly higher EE than LA rats (Figure 3D, t18 = −2.26, P = 0.035) while there were no differences in un-corrected EE between HA and LA rats averaged over IDC recording sessions (Supplementary Table 2).
Diet-induced obesity sensitivity in HA/LA rats
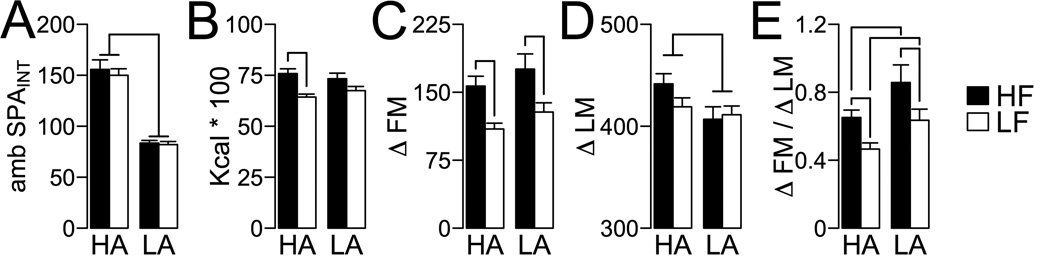
To test the contribution of ambulatory SPAINT levels to resistance to DIO, HA and LA rats (Figure 4A) were fed a high fat (HF; HA, N = 17; LA, N = 10) and low fat (LF; HA, N = 16; LA, N = 9) ad libitum for 10 weeks. Analysis of cumulative food intake (Figure 4B) with a 2-way ANOVA showed a significant effect of diet (F1,48 = 18.70, P < 0.001) and no effect of HA/LA phenotype (F1,48 = 0.006, P = 0.940) or their interaction (F1,48 = 1.58, P = 0.214). A similar pattern was observed for accumulation of fat mass (ΔFM; diet, F1,48 = 18.86, P < 0.001; HA/LA phenotype, F1,48 = 2.71, P = 0.106, [diet * HA/LA phenotype] interaction, F1,48 = 0.00, P = 0.998). Pairwise analysis indicated that for caloric intake there was increased caloric consumption due to HF diet only in HA rats (Figure 3B), while HF consumption increased ΔFM in both HA and LA rats (Figure 4C). On the contrary, for lean mass gain (ΔLM, Figure 4D) there was a significant effect of the HA/LA phenotype (F1,48 = 4.30 P = 0.043) but not of diet (F1,48 = 1.51, P = 0.22), or their interaction (F1,48 = 1.63, P = 0.21). As obesity is defined by chronic accumulation of fat mass and not by changes in overall body weight (38), severity of obesity was measured as accumulation of fat mass relative to lean mass (Figure 4E, ΔFM/ΔLM). There was a significant effect of HA/LA phenotype (F1,48 = 10.06, P = 0.002), diet (F1,48 = 12.27, P = 0.001) in ΔFM/ΔLM, but no significant interaction (F1,48 = 0.09, P = 0.759). Pairwise analysis indicated higher ΔFM/ΔLM in HA and LA rats fed a HF diet, while ΔFM/ΔLM was lower in HA compared to LA rats under a HF or a LF diet (Figure 4E). These data suggest that, independent of diet, HA rats have a higher resistance towards an obese phenotype.
Figure 4. Development of diet induced obesity in high activity (HA) and low activity (LA) rats.
(A) HA and LA rats selected for high fat (HF; HA, N = 17; LA, N = 10) and low fat (LF; HA, N = 16; LA, N = 9) feeding for 10 weeks showed no significant differences in ambulatory SPAINT at selection (HA, W = 170, P < 0.001; LA, W = 144, P < 0.001). (B) Cumulative caloric intake. (C) Fat mass gain (ΔFM). (D) Lean mass gain (ΔLM). Line: P ≤ 0.05 for main effect of phenotype (See Results). (E) Severity of obesity as measured by ratio of fat to lean mass gain (ΔFM/ΔLM) is lower in HA compared to LA rats. For plots C, D and E, the line indicates P ≤ 0.05 for pairwise comparisons corrected for multiple comparisons (See Methods, Statistical Analysis).
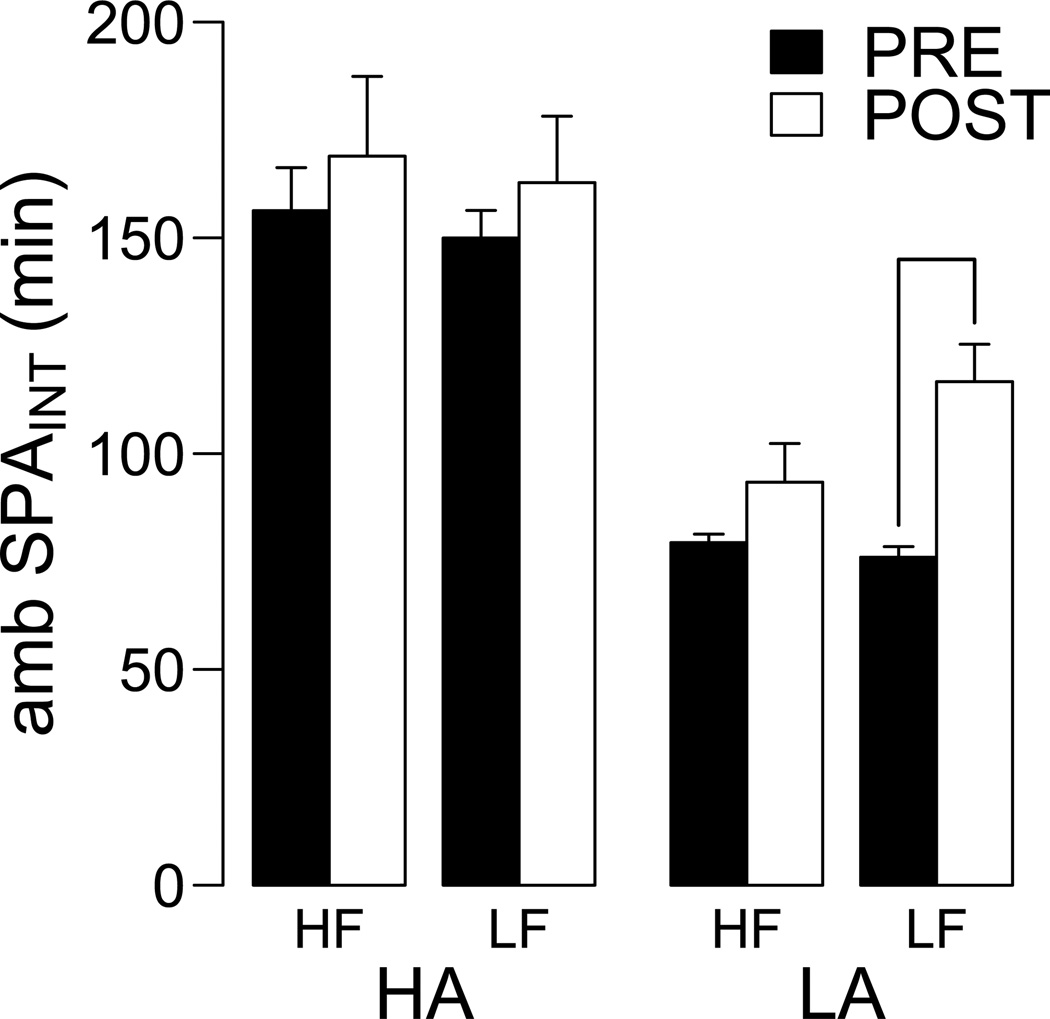
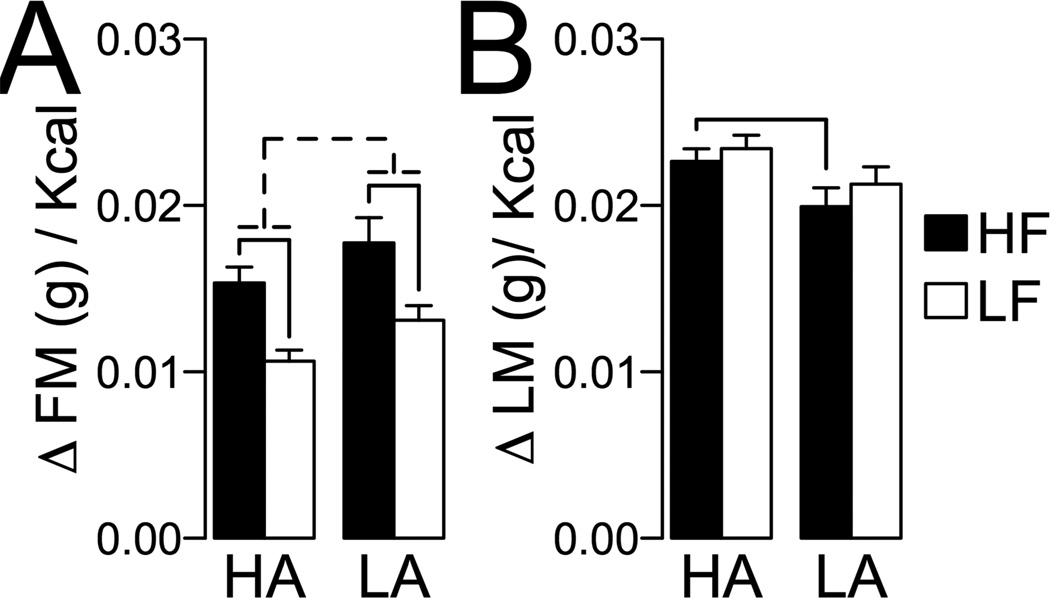
The differences in DIO severity between HA and LA rats suggest differential effects of DIO on ambulatory SPA and feeding efficiency. Figure 5 shows the effects of HF and LF feeding on 24 h ambulatory SPAINT. These data were analyzed with a repeated measures ANOVA with diet (HF or LF) and time (pre- or post-feeding) as independent variables. For HA rats, there were no significant differences in SPAINT for diet and time, and no interaction between diet and time (diet, F1,30 = 0.205, P = 0.65; time F1,30 = 0.954, P = 0.33; [diet * time] interaction, F1,30 = 0.0007, P = 0.99). For LA rats, there was no significant effect of diet (F1,11 = 2.07, P = 0.17), but there was a significant effect of time (F1,11 = 22.83, P = 0.0005) and a significant interaction between diet and time (F1,11 = 5.01, P = 0.046). Consistently, pairwise analyses indicated an increase in SPAINT in LA rats fed a LF diet (P = 0.002), and no change in SPAINT in LA rats fed a HF diet (P = 0.15). Importantly, after completion of the experiment, HA rats showed significantly higher SPAINT than LA rats, independent of diet (P < 0.05 for all pairwise comparisons). Figure 6 shows the effects of DIO on feeding efficiency for fat and lean mass (weight gain per kcal). These data were analyzed with a 2-way ANCOVA using fat or lean mass at the pre-feeding interval as a covariate. For feeding efficiency for fat mass (Figure 6A) there was a significant effect of diet (F1,47 = 29.83, P < 0.001), phenotype (F1,47 = 7.40, P = 0.009) and covariate (F1,47 = 16.86, P < 0.001) but there was no diet by phenotype interaction (F1,47 = 0.089, P = 0.76). Pairwise analyses indicated feeding efficiency for fat mass in HA and LA rats when fed a HF diet compared to LF diet (Figure 6A). For feeding efficiency for lean mass, there was a significant effect of phenotype (F1,47 = 6.68, P = 0.013), no significant effects of diet (F1,47 = 1.22, P = 0.27) or the [diet * HA/LA phenotype] interaction (F1,47 = 0.11, P = 0.74) or covariate (F1,47 = 0.049, P = 0.83). Pairwise analysis indicated that HA rats had higher feeding efficiency for lean mass compared to LA rats only under HF diet feeding (Figure 5B). Together, these results show that HA rats have lower DIO sensitivity than LA rats (Figure 4) and that different diets differentially alter SPA in HA and LA rats (Figure 5). Our results suggest resistance to obesity in HA rats is associated with a preferential ability for accumulation of lean mass under a HF diet (Figure 6B) and lower caloric efficiency for fat mass under a LF diet (Figure 6A) compared to LA rats.
Figure 5. Effect of DIO on ambulatory SPAINT in HA and LA rats.
Ambulatory SPAINT was measured pre and post 10 weeks of high fat (HF) or low fat (LF) ad libitum feeding in HA and LA rats. There were no significant effects of diet on ambulatory SPAINT in HA rats, but HF diet prevented the increase in ambulatory SPAINT observed in LA rats fed a LF diet. Although not indicated in the figure for simplicity, at completion of the study HA rats still showed significantly higher ambulatory SPAINT than LA rats, independent of diet. Line indicates P ≤ 0.05.
Figure 6. Feeding efficiency in high activity (HA) and low activity (LA) rats.
Feeding efficiency was calculated as necessary to (A) gain of fat mass (g/ kcal or (B) gain of lean mass (g)/kcal. Full line: P ≤ 0.05 for pairwise comparisons corrected for multiple comparisons (See Methods, Statistical Analysis). For (A), broken line indicates significant main effect of the HA/LA phenotype by ANCOVA (See Results).
Discussion
There is extensive evidence suggesting that modulation of orexin expression levels drives SPAINT resulting in resistance against DIO. This paper tested a critical hypothesis regarding the role of orexin in energy balance: whether naturally occurring variations in SPAINT are related to orexin gene expression and are sufficient to drive differences in DIO sensitivity. Our characterization of the HA/LA phenotype in un-manipulated SD rats shows that variations in SPAINT are: (1) related to orexin function (Figures 1 and 2) and (2) are sufficient to drive resistance to obesity (Figure 4).
To our knowledge, this is the first paper that describes the distribution of ambulatory SPAINT (Figure 1) in Sprague-Dawley rats. The reliability of the classification of SD rats as HA or LA using ambulatory SPAINT measured in an open-field (See Methods) was calculated as 72.85%. The proposed method for measurement of ambulatory SPAINT is relatively time consuming (24 h of adaptation and 24 h of recording) and thus it would be interesting to establish the minimum conditions of ambulatory SPAINT measurement to classify SD rats as HA or LA while retaining similar reliability as observed in this study.
Previous work from our laboratory in a polygenic model of obesity, the obesity-prone (OP) and obesity-resistant (OR) rats (28), showed the OR phenotype correlates with higher SPAINT (14). The reported values of SPAINT over 24 h for OP and OR rats at 1–2 months were approximately 102.2 min and 158 min respectively (14). The estimated average ambulatory 24 h SPAINT values for LA and HA rats are 71.75 and 145.5 min. Although the values are not directly comparable, in terms of SPAINT, the differences in SPAINT between groups appear to be larger in the HA/LA phenotype compared to the OP/OR model.
The HA rats have higher expression of prepro-orexin mRNA in cLH (Figure 1C) and higher orexin responsivity than LA rats to rLH and SN orexin injections (Figure 2). The higher expression of prepro-orexin mRNA in HA rats fits with previous evidence showing that down regulation of orexin signaling decreases physical activity (24, 27). We evaluated SPA after orexin-A injection in rLH and SN, as these brain sites mediate orexin effects on SPAINT. As shown previously, injection of OXA in rLH and SN increases ambulatory SPA (13), but the higher responsivity in HA compared to LA rats in ambulatory and stereotypic SPA was more robust after rLH injection than that after SN injection. This difference is intriguing as significant differences in rLH orexin-A responsivity were also observed in the OP/OR rats (14), although differential OXA responsivity for SN injections between OP and OR rats has not been reported. This suggests the rLH as an important site for the contribution of orexin signaling in controlling SPA in SD rats.
We used indirect calorimetry (IDC) to measure energy expenditure (EE) in HA and LA rats for three consecutive 24 h periods (Figure 3). In our laboratory, we use cages of the same size for routine measurements of SPA and IDC. Thus, we currently do not have an explanation for the reduction in range of ambulatory SPAINT observed during the IDC recordings (Figure 3A), compared to routine, non-IDC, SPA recordings (Figure 1A). This difference is interesting considering that rats were acclimated for 7 days before the start of the IDC recordings (See Methods) and were eating and gaining weight normally. Considering the reduction in range of ambulatory SPAINT during IDC, we classified Sprague-Dawley as HA and LA rats using the same quantiles (25% for LA and 63% for HA rats) used for classification of the HA/LA phenotype during routine SPA analysis (Figure 1A), but based on the distribution of ambulatory SPAINT as recorded in the IDC cages (Figure 3A). This resulted in a difference of ambulatory SPAINT of approximately 21 min per 24 h between HA and LA rats (Figure 3B). When correcting EE for total body weight (averaged over all three 24 h periods), HA rats have higher EE compared to LA rats (Figure 3C–D), which is estimated to be approximately 2 kcal/h. Thus, despite the reduction in range of ambulatory SPAINT, these data show that differences in ambulatory SPAINT are reflected in overall EE.
A central aspect of the usefulness of the HA/LA phenotype as a new model for obesity research depends on the robustness of the differential DIO sensitivity between HA and LA rats. Our data suggest that HA rats have lower DIO sensitivity compared to LA rats (Figure 5) and that HA rats have higher total EE than LA rats (Figure 3). Together, this suggests that differences in ambulatory and stereotypic SPAINT contribute to their DIO resistance. It is important to note that sensitivity to DIO obesity is most likely to be a multi-factorial process, in which SPAINT plays a significant role, but is clearly not the only mechanism involved. Thus, the HA/LA phenotype might prove useful in establishing how variations in SPAINT are correlated with other aspects of the development of obesity.
Under both HF and LF diet feeding, the feeding efficiency in HA rats is reflective of a tendency for a leaner phenotype. When fed a HF or LF diet, HA rats are less efficient in accumulating fat mass relative to LA rats (Figure 4A). And during HF diet feeding, HA rats are more efficient in accumulating lean mass (Figure 4B). Together, these results suggest that during HF diet feeding, HA rats adapt to caloric excess by increasing accumulation of lean mass. Body composition was measured using quantitative magnetic resonance, thus we suggest the increase in lean mass observed in HA rats represents an increase in lean muscle (29, 30), a tissue that is more metabolically demanding than white adipose tissue.
Finally, we measured ambulatory SPAINT before and after HF or LF feeding (Figure 5). Our data show no effect of diet on SPAINT of HA rats. However, LA rats fed a LF diet showed an increase in SPAINT, whereas there was no change in SPAINT in LA rats fed a HF diet. After feeding, and independent of diet, HA rats still showed higher SPAINT than LA rats. These data suggest an interaction between the HA/LA phenotype and effects of age and DIO on ambulatory SPAINT. Longitudinal studies in Sprague-Dawley rats showed increased ambulatory SPAINT between two and four months of age (9), which matches the time frame used in our studies. This suggests that in LA rats, HF consumption prevents the natural increase in SPAINT at this developmental stage.
In summary, these studies describe a new model of differential DIO sensitivity, the HA and LA rats. This model is based on naturally occurring variations in SPAINT in male SD rats. These data further suggest that differences in orexin signaling contribute to the observed variations in SPAINT and DIO sensitivity. This model should prove useful in future studies that address how differences in orexin and other neuromodulators might contribute to individual sensitivity to obesity.
Supplementary Material
Acknowledgments
Support: Department of Veterans Affairs, National Institute of Diabetes and Digestive and Kidney diseases Grant DK078985, and the Minnesota Obesity Center.
Footnotes
Disclose Summary: The authors declare no conflict of interests.
Supplementary Material is available at www.nature.com/obesity
REFERENCES
- 1.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990 May 24;322(21):1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 2.Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr. 1986 Jul;56(1):1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 3.Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, et al. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E251–E260. doi: 10.1152/ajpendo.00401.2007. [DOI] [PubMed] [Google Scholar]
- 4.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996 Jul;60(1):37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 5.Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J Nutr. 2004 Dec;134(12):3264–3269. doi: 10.1093/jn/134.12.3264. [DOI] [PubMed] [Google Scholar]
- 6.Hesse D, Dunn M, Heldmaier G, Klingenspor M, Rozman J. Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet- induced obesity. Physiol Behav. 2010 Mar 3;99(3):370–380. doi: 10.1016/j.physbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999 Jan 8;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 8.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005 Jan 28;307(5709):584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 9.Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. International journal of obesity. 2011 May 24; doi: 10.1038/ijo.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R699–R710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 11.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011 Jan 15;214(Pt 2):206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002 Mar 15;104(1–3):27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 13.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, et al. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006 Sep 29;142(1):29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006 Oct;291(4):R889–R899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 15.Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87(2):71–90. doi: 10.1159/000110802. [Review] [DOI] [PubMed] [Google Scholar]
- 16.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998 Jan 6;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998 Mar 6;92(5):1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- 18.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998 Dec 1;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taheri S, Gardiner J, Hafizi S, Murphy K, Dakin C, Seal L, et al. Orexin A immunoreactivity and preproorexin mRNA in the brain of Zucker and WKY rats. Neuroreport. 2001 Mar 5;12(3):459–464. doi: 10.1097/00001756-200103050-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002 Mar 15;104(1–3):131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 21.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 22.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001 Jun 18;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 23.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998 Oct 30;438(1–2):71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 24.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001 May;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 25.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009 Jan 7;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004 Apr;286(4):E551–E559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 27.Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011 Mar;25(1–2):52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997 Aug;273(2 Pt 2):R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 29.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010 Aug;18(8):1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obesity research. 2004 Jan;12(1):150–160. doi: 10.1038/oby.2004.20. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Amsterdam; Boston: Elsevier Academic Press; 2005. [Google Scholar]
- 32.Kotz CM, Levine AS, Billington CJ. Effect of naltrexone on feeding, neuropeptide Y and uncoupling protein gene expression during lactation. Neuroendocrinology. 1997 Apr;65(4):259–264. doi: 10.1159/000127183. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May 1;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team DC. R: A language and environment for statistical computing. 2.11.1 ed. Vienna, Austria: ISBN; 2010. [Google Scholar]
- 35.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988 Dec;75(4):800–802. [Note] [Google Scholar]
- 36.Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. International journal of obesity. 2006 Sep;30(9):1322–1331. doi: 10.1038/sj.ijo.0803280. [Review] [DOI] [PubMed] [Google Scholar]
- 37.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011 Jan;60(1):17–23. doi: 10.2337/db10-0909. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.