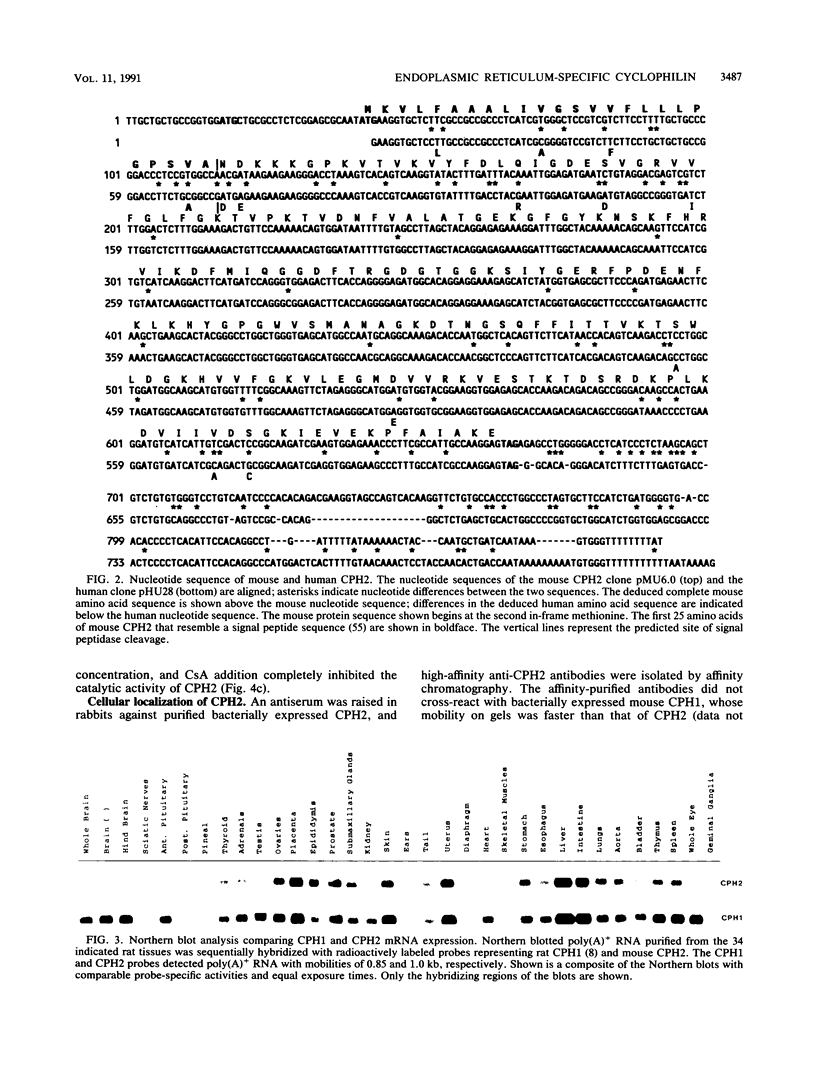
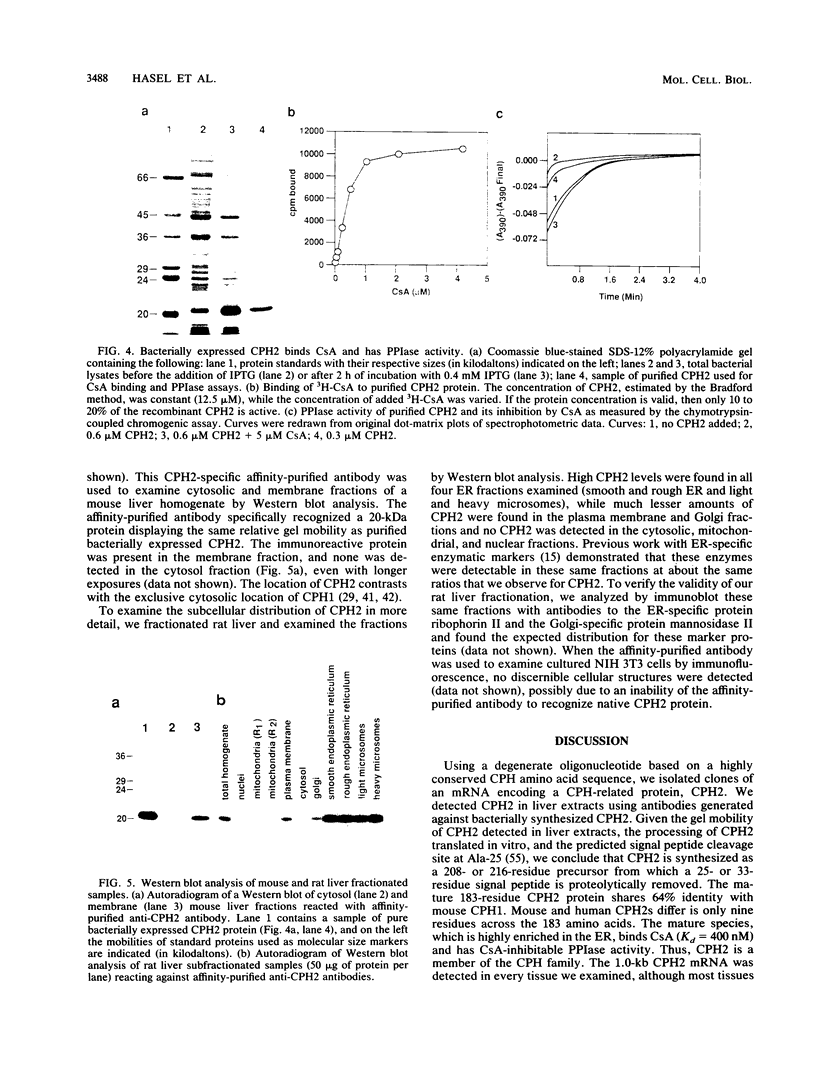
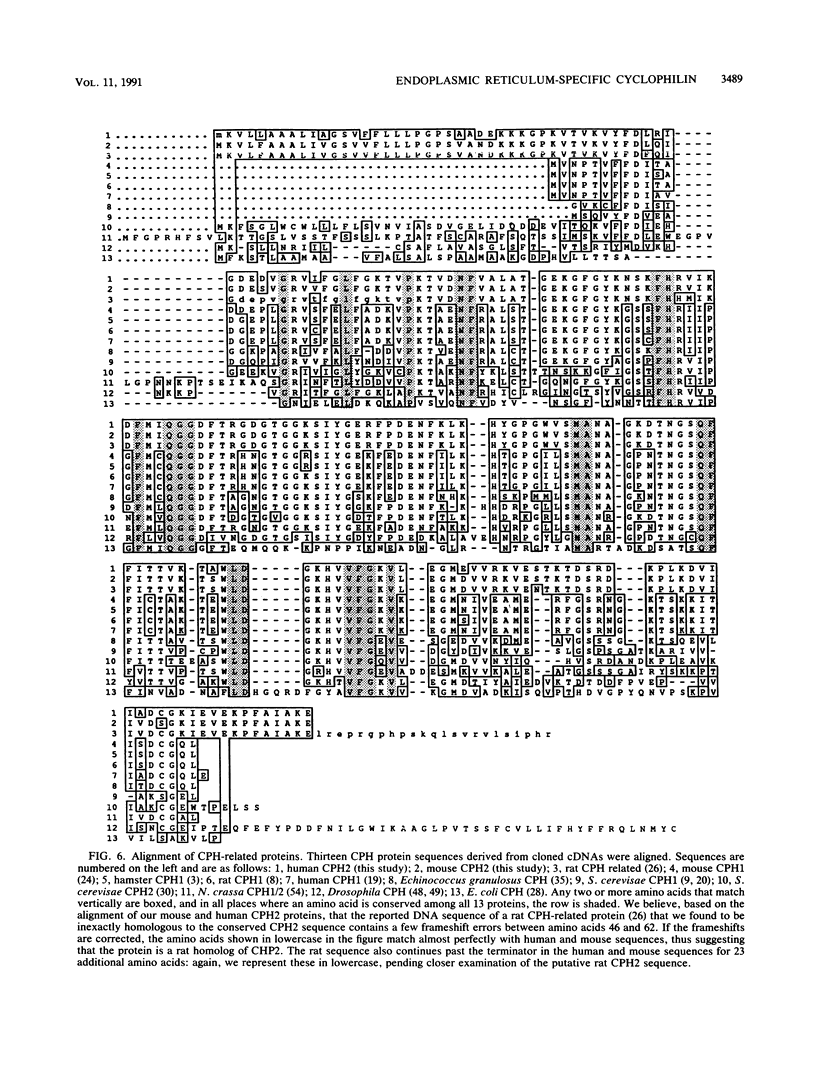
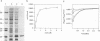Abstract
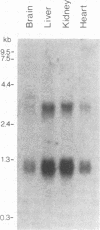
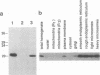
Cyclophilin is a ubiquitously expressed cytosolic peptidyl-prolyl cis-trans isomerase that is inhibited by the immunosuppressive drug cyclosporin A. A degenerate oligonucleotide based on a conserved cyclophilin sequence was used to isolate cDNA clones representing a ubiquitously expressed mRNA from mice and humans. This mRNA encodes a novel 20-kDa protein, CPH2, that shares 64% sequence identity with cyclophilin. Bacterially expressed CPH2 binds cyclosporin A and is a cyclosporin A-inhibitable peptidyl-prolyl cis-trans isomerase. Cell fractionation of rat liver followed by Western blot (immunoblot) analysis indicated that CPH2 is not cytosolic but rather is located exclusively in the endoplasmic reticulum. These results suggest that cyclosporin A mediates its effect on cells through more than one cyclophilin and that cyclosporin A-induced misfolding of T-cell membrane proteins normally mediated by CPH2 plays a role in immunosuppression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R., Majdic O., Meingassner J. G., Knapp W. In vitro effects of cyclosporin A (CSA) on human hemopoietic cell lines. Immunopharmacology. 1982 Dec;5(2):123–127. doi: 10.1016/0162-3109(82)90043-1. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Sylvester D. A Chinese hamster ovary cyclophilin cDNA sequence. Nucleic Acids Res. 1990 Jan 11;18(1):200–200. doi: 10.1093/nar/18.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Crimaudo C., Hortsch M., Gausepohl H., Meyer D. I. Human ribophorins I and II: the primary structure and membrane topology of two highly conserved rough endoplasmic reticulum-specific glycoproteins. EMBO J. 1987 Jan;6(1):75–82. doi: 10.1002/j.1460-2075.1987.tb04721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dietmeier K., Tropschug M. Nucleotide sequence of a full-length cDNA coding for cyclophilin (peptidyl-prolyl cis-trans isomerase) of Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Jan 25;18(2):373–373. doi: 10.1093/nar/18.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H. The refolding of urea-denatured ribonuclease A is catalyzed by peptidyl-prolyl cis-trans isomerase. Biochim Biophys Acta. 1985 Mar 22;828(1):39–42. doi: 10.1016/0167-4838(85)90006-8. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Gerace L., Ottaviano Y., Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982 Dec;95(3):826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Hasel K. W., Sutcliffe J. G. Nucleotide sequence of a cDNA coding for mouse cyclophilin. Nucleic Acids Res. 1990 Jul 11;18(13):4019–4019. doi: 10.1093/nar/18.13.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman R. J., Hultsch T. Cyclosporin A: new insights for cell biologists and biochemists. New Biol. 1990 Aug;2(8):663–672. [PubMed] [Google Scholar]
- Iwai N., Inagami T. Molecular cloning of a complementary DNA to rat cyclophilin-like protein mRNA. Kidney Int. 1990 Jun;37(6):1460–1465. doi: 10.1038/ki.1990.136. [DOI] [PubMed] [Google Scholar]
- Kahan B. D., Yoshimura N., Pellis N. R., Burstain T., Van Buren C. T., Leinikki P., Schreiber R. D., Roesel T. R., Kerman R. H. Pharmacodynamics of cyclosporine. Transplant Proc. 1986 Dec;18(6 Suppl 5):238–251. [PubMed] [Google Scholar]
- Kawamukai M., Matsuda H., Fujii W., Utsumi R., Komano T. Nucleotide sequences of fic and fic-1 genes involved in cell filamentation induced by cyclic AMP in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4525–4529. doi: 10.1128/jb.171.8.4525-4529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Koser P. L., Sylvester D., Livi G. P., Bergsma D. J. A second cyclophilin-related gene in Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Mar 25;18(6):1643–1643. doi: 10.1093/nar/18.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X. Protein-disulphide isomerase and prolyl isomerase act differently and independently as catalysts of protein folding. Nature. 1988 Feb 4;331(6155):453–455. doi: 10.1038/331453a0. [DOI] [PubMed] [Google Scholar]
- Lightowlers M. W., Haralambous A., Rickard M. D. Amino acid sequence homology between cyclophilin and a cDNA-cloned antigen of Echinococcus granulosus. Mol Biochem Parasitol. 1989 Oct;36(3):287–289. doi: 10.1016/0166-6851(89)90177-1. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Chen C. M., Schreiber S. L., Walsh C. T. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker M. P., Merker M. M., Handschumacher R. E. Uptake and nature of the intracellular binding of cyclosporin A in a murine thymoma cell line, BW5147. J Immunol. 1984 Jun;132(6):3064–3070. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Ross J., Newton L., Luetscher J., Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984 Sep 13;311(11):699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Shortridge R. D., Larrivee D. C., Ono T., Ozaki M., Pak W. L. Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporine A binding protein). Proc Natl Acad Sci U S A. 1989 Jul;86(14):5390–5394. doi: 10.1073/pnas.86.14.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Bruckner P., Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem. 1991 Jan 15;266(2):1299–1303. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]