Figure 7. Protein Phosphatase 1 Modulates CLOCK/BMAL1 Function.
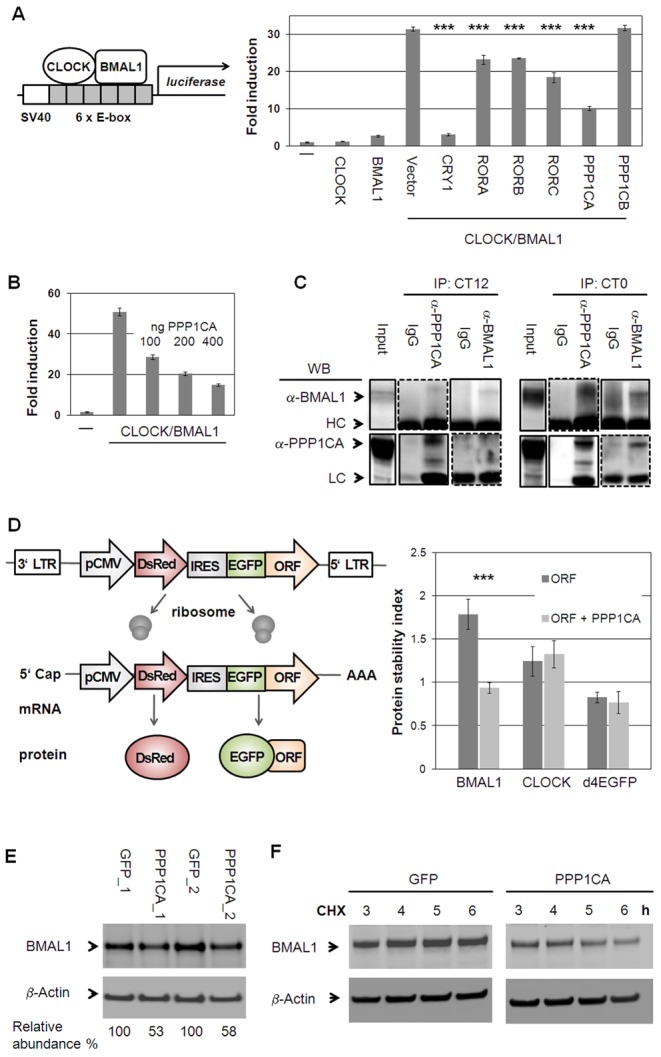
(A) CLOCK and BMAL1 interactors identified in yeast and their paralogs were co-transfected with CLOCK/BMAL1 and an E-box containing luciferase reporter (see also Figure S7A). Shown are means ± s.d. of CLOCK/BMAL1 modifiers (n = 3; *** p<0.001, t-test). (B) PPP1CA dose-dependently reduces CLOCK/BMAL1 transactivation (n = 3; means ± SD.). (C) PPP1CA is present in the CLOCK/BMAL1 complex. Murine livers were harvested at indicated times. Dashed lines: longer exposure. (LC: light chain; HC: heavy chain). (D) PPP1CA destabilizes BMAL1 protein. Left: Stability is reported by the change of EGFP to DsRed ratio [30], [31]. Right: PPP1CA co-expression with BMAL1, CLOCK or short-lived EGFP fusion proteins in U2OS cells reduces BMAL1 stability (mean ± s.d.; ***p<0.001; t-test; n = 3; (see also Figure S7B, S7C). (E) Endogenous BMAL1 levels are reduced upon PPP1CA overexpression in U2OS cells. Depicted are two independent experiments. (F) PPP1CA reduces BMAL1 stability. U2OS cells stably expressing PPP1CA or GFP were harvested at the indicated time points after cycloheximide (CHX) application and protein levels were analyzed by Western blot. Shown is one representative of two independently performed experiments (see also Figure S7D).
