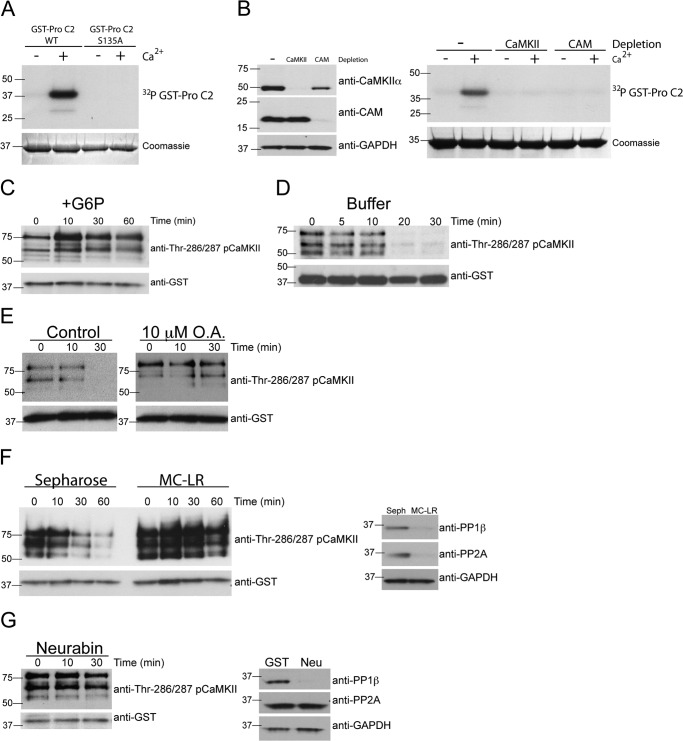
FIGURE 5.
A metabolically regulated factor inhibits PP1 dephosphorylation of CaMKII. A, CaMKII bound to caspase 2 is capable of phosphorylating caspase 2. GST-caspase 2 pro-domain (Pro C2) (WT or S135A) bound to glutathione-Sepharose was incubated in egg extract to bind CaMKII. GST-Pro C2 bound to endogenous CaMKII was retrieved, washed, and incubated in kinase buffer containing [γ-32P]ATP in the absence or presence of 500 μm CaCl2. Beads were washed, eluted, and analyzed for GST-Pro C2 phosphorylation by SDS-PAGE, Coomassie Blue staining, and autoradiography. n = > 3 independent experiments. B, CaMKII and calmodulin are required for Ca2+-induced phosphorylation of caspase 2. CaMKII or calmodulin (CAM) were depleted from egg extracts using CAM-Sepharose or calmodulin binding peptide-Sepharose, respectively. Control depletions were carried out with Sepharose alone. Left panel, selective depletion of CaMKII, and CAM was confirmed by immunoblotting for CaMKIIα, CAM, and GAPDH as a loading control. Right panel, GST-Pro C2 bound to glutathione-Sepharose was incubated in control, CaMKII-depleted, or CAM-depleted egg extracts in the absence or presence of 500 μm CaCl2 and [γ-32P]ATP. Beads were washed and analyzed as in A. n = > 3 independent experiments. C, caspase 2 binds active CaMKII in the presence of G6P. GST-Pro C2 bound to glutathione-Sepharose was incubated in egg extract in the presence of G6P. At the indicated times, beads were collected, washed, eluted, and immunoblotted for pCaMKII Thr-286/287 and GST as a loading control. n = > 3 independent experiments. D, CaMKII bound to caspase 2 is dephosphorylated rapidly when removed from egg extract. GST-Pro C2 bound to glutathione-Sepharose was incubated in egg extract containing G6P. Beads were then collected, washed, and incubated in phosphatase buffer. At the indicated times, beads were collected and analyzed for pCaMKII Thr-286/287 as in C. n = > 3 independent experiments. E, inhibition of PP1 and PP2A with okadaic acid inhibits dephosphorylation of endogenous CaMKII bound to caspase-2. Left panel, GST-Pro C2 bound to glutathione-Sepharose was incubated in egg extracts and G6P in the presence of 10 μm okadaic acid. Beads were collected, washed, and incubated in phosphatase buffer in the absence or presence of 10 μm okadaic acid. At the indicated times, beads were collected and analyzed for pCaMKII Thr-286/287 as in C. n = 3 independent experiments. F, depletion of PP1 and PP2A inhibits dephosphorylation of endogenous CaMKII bound to caspase 2. Left panel, GST-Pro C2 bound to glutathione-Sepharose was incubated in cytosolic fractions of egg extract depleted with Sepharose or microcystin-Sepharose (MC-LR) in the presence of G6P. Beads were collected, washed, and then incubated in phosphatase buffer. At the indicated times, beads were collected and analyzed for pCaMKII Thr-286/287 as in C. Right panel, selective depletion of PP1 and PP2A was confirmed by immunoblotting for PP1β, PP2A, and GAPDH as a loading control. n = 3 independent experiments. G, depletion of PP1 inhibits dephosphorylation of endogenous CaMKII bound to caspase 2. Left panel, GST-Pro C2 bound to glutathione-Sepharose was incubated in egg extract depleted with GST-neurabin in the presence of G6P. Beads were collected, washed, and incubated in phosphatase buffer. At the indicated times, beads were collected and analyzed for pCaMKII Thr-286/287 as in C. Right panel, selective depletion of PP1, but not PP2A, was confirmed by immunoblotting for PP1β, PP2A, and GAPDH as a loading control. n = 3 independent experiments.