FIGURE 4.
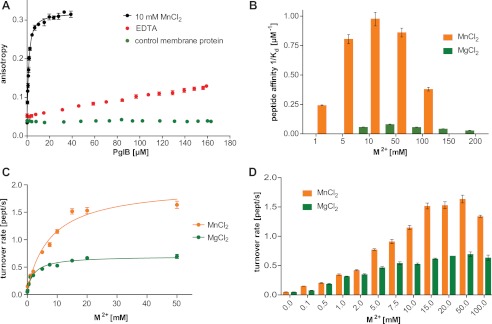
Metal ion dependence of in vitro peptide binding and glycosylation. A, fluorescence anisotropy experiments using fluorescently labeled peptide containing the DQNAT sequon, performed as in Fig. 2B but testing the role of MnCl2. A control measurement with an unrelated membrane protein prepared in the same detergent is shown. The WT data are identical to that shown in Fig. 2B. B, peptide binding affinities (1/Kd) as a function of MnCl2 and MgCl2 concentrations, respectively. Each bar indicates a single fluorescence anisotropy curve (supplemental Fig. S3), error bars indicate the S.E. of the obtained fit for each dataset. C, turnover rates of WT PglB (4 nm) and fluorescently labeled peptide (10 μm) containing the DQNAT sequon as a function of Mn2+ and Mg2+ concentrations. Apparent dissociation constants for metal ions were obtained by fitting the data using a single binding site model (R2(MnCl2) = 0.981; R2(MgCl2) = 0.981). D, overview of glycosylation turnover rates at different concentrations of MnCl2 and MgCl2 (C and D). Each data point reflects a turnover rate determination as shown in Fig. 2F, error bars indicate the S.E. of each fit.
