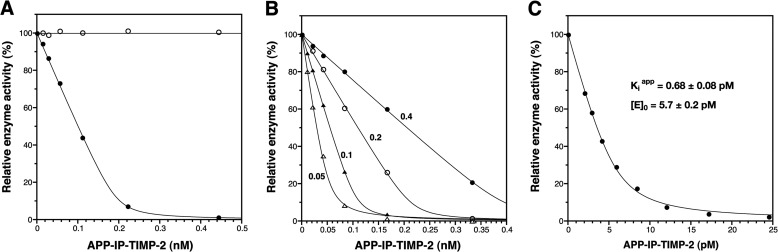
FIGURE 2.
APP-IP-TIMP-2 inhibition of peptidolytic activity of MMP-2. A, the active form of MMP-2 (0.2 nm, ●) and the hemopexin-like domainless form of MMP-2 (0.2 nm, ○) were incubated with 50 μm 3163v at 37 °C for 30 min in the presence of indicated concentrations of APP-IP-TIMP-2. B, four different concentrations of MMP-2 (0.4 nm, ●; 0.2 nm, ○; 0.1 nm, ▴; 0.05 nm, ▵) were incubated with 50 μm 3163v at 37 °C for 15, 30, 60, and 120 min, respectively, in the presence of the indicated concentrations of APP-IP-TIMP-2. C, MMP-2 (6.0 pm) was incubated with 50 μm 3163v at 37 °C for 4 h in the presence of the indicated concentrations of APP-IP-TIMP-2 as described under “Experimental Procedures.” All reaction mixtures contained TBS-Ca-Brij and 0.01% bovine serum albumin. The amount of 3163v hydrolyzed by MMP-2 in the absence of APP-IP-TIMP-2 was taken as 100%. The enzyme activity is shown as the relative amount of 3163v hydrolyzed by the enzyme on the ordinate. The curve fitting of each data was carried out using Equation 1 as described under “Experimental Procedures.” The Kiapp and [E]0 values shown in C were obtained by computer-assisted non-linear curve fitting.