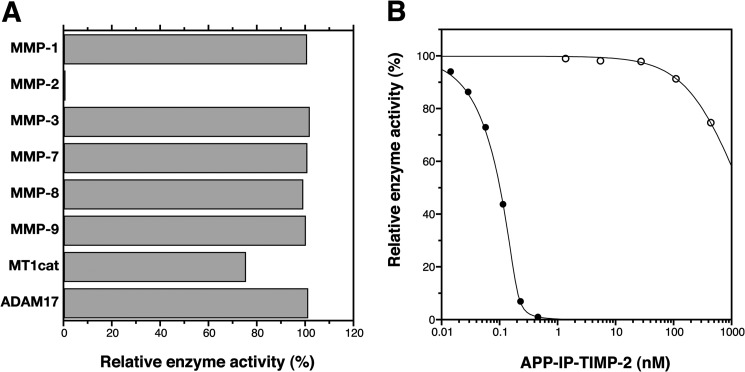
FIGURE 3.
Enzyme specificity in inhibitory activity of APP-IP-TIMP-2. A, MMP-1 (0.8 nm), MMP-2 (0.2 nm), MMP-3 (27 nm), MMP-7 (1.5 nm), MMP-8 (7.2 nm), MMP-9 (1.4 nm), and the catalytic domain of MT1-MMP (MT1cat, 1.0 nm) were incubated with 50 μm 3163v, and ADAM17 (2.4 nm) was incubated with 50 μm 3226v at 37 °C for 30 min in the absence or presence of APP-IP-TIMP-2 (420 nm). B, MMP-2 (0.2 nm, ●) and the catalytic domain of MT1-MMP (1.0 nm, ○) were incubated with 50 μm 3163v at 37 °C for 30 min in the presence of the indicated concentrations of APP-IP-TIMP-2. The amount of the peptide substrate (3163v or 3226v) hydrolyzed in the absence of APP-IP-TIMP-2 was taken as 100%. The enzyme activity is shown as the relative amount of the substrate hydrolyzed by the enzyme. The curve fitting of each data in B was carried out using Equation 1 as described under “Experimental Procedures.”