Abstract
Brahmi Ghrita, a polyherbal Ayurvedic formulation is recommended in the management of various psychological disorders like Unmada, Apasmara and Graharogas. The present study deals with the pharmacognostical identification of ingredients of Brahmi Ghrita and its physico-chemical analysis. Pharmacognostical study containing both macroscopic and powder microscopy of raw drug revealed the quality and genuineness of all the constituents of Brahmi Ghrita. Organoleptic features of coarse powder made out of the crude drugs were within the standards prescribed. Acid value was 0.16075, saponification value 184.17, Refractive Index value 1.467 at room temperature, Iodine value 26.715, Specific gravity at room temperature was 0.9133. HPTLC was carried out after organizing appropriate solvent system in which maximum 9 spots were distinguished and most of the Rf values were identical in alcoholic extract which shows the presence of certain definite constituents in Brahmi Ghrita.
Keywords: Brahmi Ghrita, HPTLC, pharmacognosy, physico-chemical analysis
Introduction
Brahmi being a Medhya drug is recommended for various psychosomatic and psychiatric disorders. Most of the formulations acting on psyche are ghee based. It is well established that, the drugs to have its action on brain should have the capacity to cross the blood–brain barrier and for that purpose ghee is the best drug vehicle. Brahmi Ghrita is recommended for the management of Unmada (Insanity), Alakshmi (Inauspicious), Apasmara (Epilepsy), Papavikaras (Diseases due to sinful acts),[1] and for Apasmara, Unmada, Graha Rogas (Diseases afflicted by evil spirits).[2]
The objectives of adulteration are 4-fold, namely, to increase the bulk or weight of the substance, to improve its appearance, to give it a false strength, or to rob it of its most valuable constituents. The recognition of such impurities, and the tracing of them to their source, is of prime importance in pursuing a charge of adulteration.[3] The objective of the present study is to ascertain the genuinity of all the ingredients of Brahmi Ghrita and presence of components as recommended in Ayurvedic Pharmacopoeia of India (API) –Brahmi,[4] Vacha,[5] Shankhapushpi,[6] Kushtha[7] through pharmacognostical and physico-chemical studies.
Aims and objectives
Pharmacognostical study of individual components of Brahmi Ghrita.
Physico-chemical analysis of Brahmi Ghrita.
Materials and Methods
Collection and authentication of raw drugs
Whole plant of Brahmi (Bacopa monnieri (L.) Pennel) was collected from Foundation for Revitalization of Local Health Traditions (FRLHT), Bangalore in the month of December 2011. Other ingredients of Brahmi Ghrita [Table 1] were procured from Pharmacy, Gujarat Ayurved University. All these were identified and authenticated in Pharmacognosy Laboratory, IPGT and RA, Gujarat Ayurved University, Jamnagar. Ghrita (Cow’s ghee) was procured from Khadi Gramodyoga Bhandar, Jamnagar and Brahmi Ghrita[1] was prepared in Pharmacy of Gujarat Ayurved University, Jamnagar.
Table 1.
Ingredients of Brahmi Ghrita
Method of preparation of Brahmi Ghrita
Brahmi Swarasa was extracted by exerting mechanical pressure on fresh Brahmi. In a large vessel Go-Ghrita was poured, when it liquefies under moderate flame, Kalka of Vacha, Kushtha, Shankhapushpi made in Brahmi Swarasa was added, followed by addition of Brahmi Swarasa. To get final product, the contents were subjected to heat till up to Sneha Siddhi Lakshanas were observed.[8]
Pharmacognostical evaluation of ingredients of Brahmi Ghrita
Organoleptic study
Individual powders were subjected for various sensory characters like color, taste odor, etc., and were carefully noted down.[9]
Powder microscopy
The powders of respective parts of [Table 1] Brahmi, Vacha, Kushtha, Shankhapushpi were studied separately with and without staining. The microphotographs were taken under Corlzeiss binocular microscope attached with camera.[10, 11]
Physico-chemical study
Brahmi Ghrita was analyzed using various standard physicochemical parameters such as Acid value, saponification value, Refractive Index value, iodine value, specific gravity. High Performance Thin Layer Chromatography (HPTLC) was carried out after making appropriate solvent system with methanolic extract of Brahmi Ghrita[12] at Pharmaceutical chemistry laboratory, IPGT and RA, Jamnagar.
High Performance Thin Layer Chromatography
Preparation of sample solution
The Ghrita sample was adsorbed on silica gel. The mixture was extracted with hexane. Hexane fraction was discarded. The material was extracted with methanol. Process was repeated for 3 times. The methanol layer was collected, filtered, and evaporated off. The dried material was again dissolved in methanol and used for Thin Layer Chromatography (TLC) identification.
Chromatographic conditions
Stationary phase: Silica gel GF 254(E. Merck) precoated TLC plates
Mobile phase: Dichloromethane: methanol: water (4.5:1.0:0.1 v/v/v)
Sample volume: 5 μl
Sample for HPTLC: Methanol extract of Brahmi Ghrita
Spray reagent: Vaniline-sulfuric acid
Instrumental conditions
Camag HPTLC instrument catalog No 0276481(Switzerland) was used for experiment
Application mode: Camag Linomat V
Development chamber: Camag twin trough chamber.
Plates: Precoated silica gel GF254 plates.
Chamber saturation: 30 min.
Development time: 30 min.
Development distance: 7 cm.
Scanner: Camag scanner III.
Detection: Deuterium lamp, Tungsten lamp
Data System: Win cats software
Procedure
Before spotting, the plates were prewashed with methanol. Sample solutions were applied to the plates as sharp bands by means of Camag Linomat V sample applicator. The spots were dried in a current of air. The mobile phase (20 ml) was poured into a twin trough glass chamber whole assembly was left to equilibrate for 30 min and the plate was placed in the chamber. The plate was then developed until the solvent front had travelled at a distance of 80 mm above the base of plate. The plate was then removed from chamber and dried in a current of air. Detection and quantification was performed with Camag TLC scanner 3 at a wavelength of 254 and 366 nm.
Observations and Results
Pharmacognostical analysis
Organoleptic characters were noted down and are depicted in Table 2.
Table 2.
Organoleptic characters of ingredients of Brahmi Ghrita

Microscopic characters: Powder microscopy of Brahmi Ghrita ingredients was studied and microphotographs were placed at respective figures.
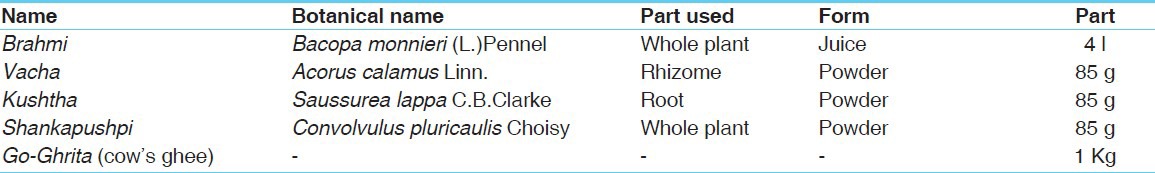
Kushtha: Fiber, prismatic crystals, tannin, cork in surface, annular, and pitted vessels [Figure 1a–d].
Figure 1.
Powder microscopy of Brahmi Ghrita ingredients Kushtha (a) Prismatic crystals (b) Tannin (c) Annular vessels (d) Pitted vessels
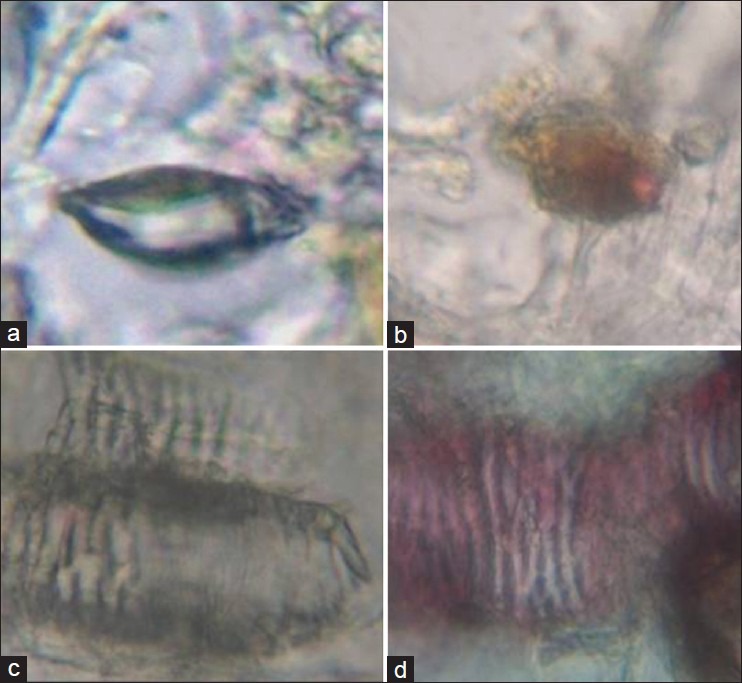
Brahmi: Prismatic crystals, fiber, fragments of annular vessels, stomata, fragments of pallside parenchyma, tannin contents, and fragments of pitted vessels [Figure 2a–d].
Figure 2.
Brahmi (a) Prismatic crystals (b) Fragments of annular vessels (c) stomata (d) Fragments of pitted vessels
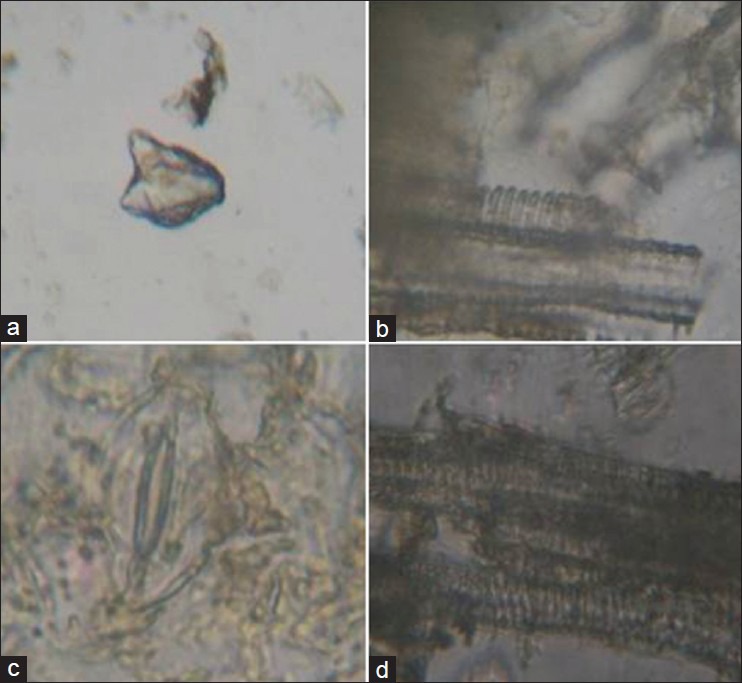
Vacha: Tannin, simple starch grains, oleoresins, parenchyma cells with starch grains, fiber with lumen, fragments of annular and pitted vessels [Figure 3a–d].
Figure 3.
Vacha (a) Olioresins (b) Parenchyma cells with starch grains (c) Fiber with lumen (d) Fragments of annular and pitted vessels
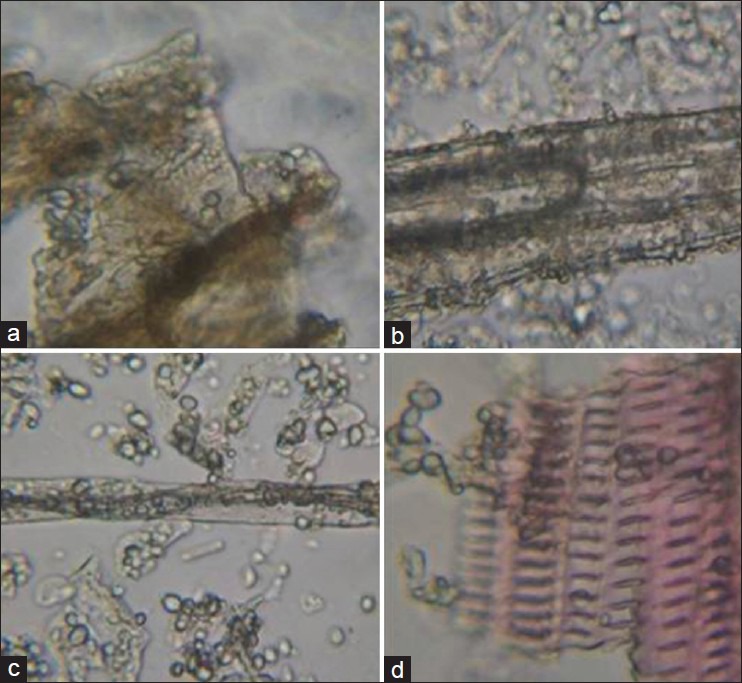
Shankhapushpi: Simple unicellular trichome, stellate trichome, prismatic crystals, fragments of spiral vessels, lignified fibers, stomata, fragments of pitted vessels, tannin, starch grains in group [Figure 4a–d].
Figure 4.

Shankhapushpi (a) Stellate trichome, (b) Prismatic crystals (c) Stomata (d) Fragments of pitted vessels
Physico-chemical analysis
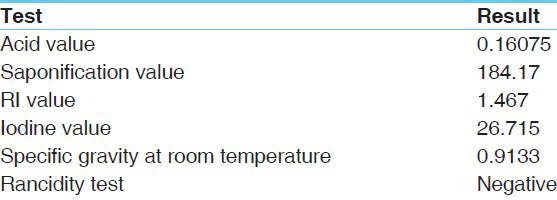
Brahmi Ghrita was analyzed using various standard physico-chemical parameters such as acid value, saponification value, RI value, iodine value, specific gravity [Table 3].
Table 3.
Physicochemical parameters
High Performance Thin Layer Chromatography
On analyzing under densitometer at 254 nm, the chromatogram showed 9 peaks, while at 366 nm the chromatogram showed 6 peaks. And after spray the chromatogram showed 8 peaks. [Table 4], [Figure 5a–c]. Three dimensional (3d) densitogram at 254 nm shows comparative Rf value of sample with standard [Figure 6a–c].
Table 4.
High performance thin layer chromatography
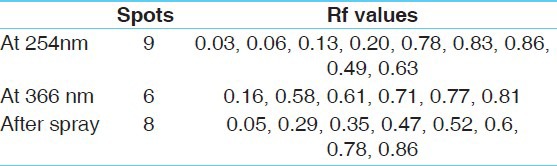
Figure 5.

HPTLC figure prints (a) at 254 nm (b) at 366 nm (c) After sprays T1-Brahmi Ghrita sample
Figure 6.
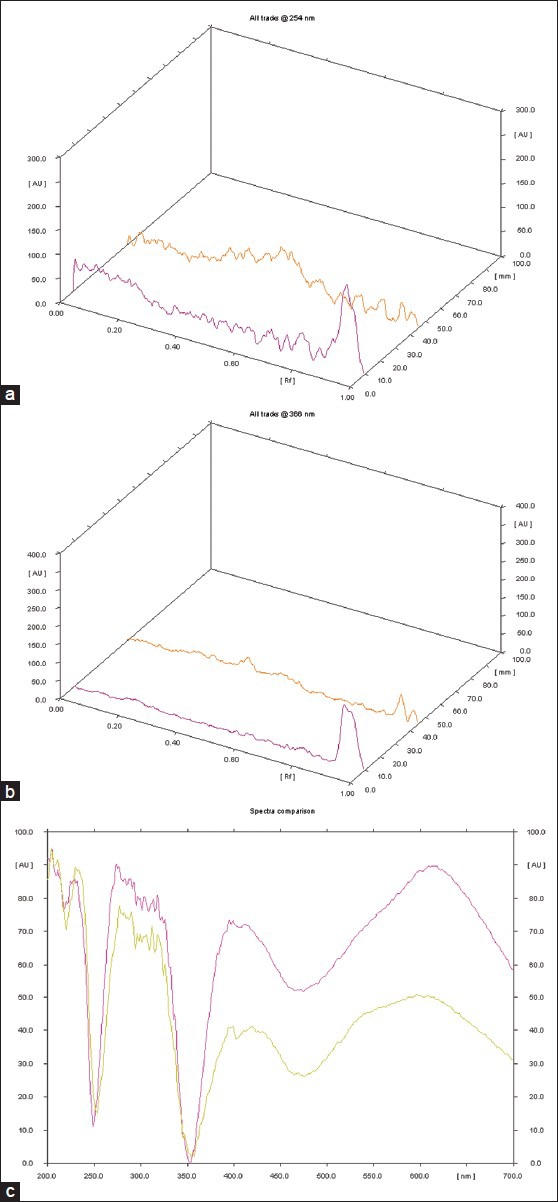
(a) 254 3D (b) 366 nm (c) Spectral comparison at Rf 0.78
Discussion
Pharmacognostical study reveals authentification of individual raw drugs of Brahmi Ghrita and is cross verified.[3-6] The oleoresins, pitted vessels, tannin, prismatic crystals, stomata, fiber are observed in ingredients. All the physico-chemical parameters, acid value, saponification value, RI value, iodine value, specific gravity analyzed were within the normal reference range.[13] In HPTLC one spot was detected at Rf value 0.78, which indicates presence of Bacoside in Brahmi Ghrita.[14] All the results show that the prepared Ghrita formulation is not rancid (after 10 months of preparation) and the quality of the Ghrita is standard.
Conclusion
Pharmacognostical study findings confirm the ingredients present in the Brahmi Ghrita. Identified phytochemical components like bacoside support the intended action of the formulation. Under densitometer at 254 nm 9 peaks and under 366 nm 6 peaks were found and after spray, 8 peaks were found. It is inferred that the formulation meets maximum qualitative standards. The results of this study may be used as the reference standard in further research undertakings of its kind.
References
- 1.Agnivesha, Charaka, Dridhabala . Charaka samhita, Chikitsa stana, Apasmara chikitsa adhyaya, 10/25. editor 2009. [Google Scholar]; Acharya YT. Varanasi: Chaukamba Prakashana; p. 475. [Google Scholar]
- 2.Bhavamishra . Bhavaprakasha, uttarardha 23 / 18. Varanasi: Choukhamba Samskruta Sansthana; 2005. p. 225. [Google Scholar]
- 3.Adulteration [Last accessed on 2012 Apr 27]. Available from: http://www.1902 encyclopedia.com/A/ADU/Adulteration.html .
- 4.Anonymous. The Indian Pharmacopoeia. Vol. 2. New Delhi: Govt of India publication; 1996. pp. 25–6. [Google Scholar]
- 5.Anonymous. The Indian Pharmacopoeia. Vol. 2. New Delhi: Govt of India publication; 1996. pp. 25–6. [Google Scholar]
- 6.Anonymous. The Indian Pharmacopoeia. Vol. 2. New Delhi: Govt of India publication; 1996. pp. 25–6. [Google Scholar]
- 7.Anonymous. The Indian Pharmacopoeia. Vol. 2. New Delhi: Govt of India publication; 1996. pp. 25–6. [Google Scholar]
- 8.Sharangadhara . Sharangadhara Samhita. Madhyama Khanda, Snehakalpana Adhyaya 9/6. editor 2009. [Google Scholar]; Shailaja Shreevatsav. Varanasi: Chaukhamba Orientalia; p. 216. [Google Scholar]
- 9.Wallis TE. Text Book of Pharmacognosy. 5th ed. New Delhi: CBS Publishers; 1985. pp. 571–8. [Google Scholar]
- 10.Khandelwal KR. Practical Pharmacognosy. Pune: Nirali Prakashan; 2008. pp. 149–66. [Google Scholar]
- 11.Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 42nd ed. Pune: Nirali Prakashan; 2008. p. Chap 61, A 1. [Google Scholar]
- 12.Stahl E. Thin layer chromatography a laboratory hand book. Berlin: Springer-Verlag; 1969. pp. 124–241. [Google Scholar]
- 13.Manual of methods of analysis of food oils and fats. New Delhi: Directorate General of Health Services Ministry of Health and Family Welfare Government of India; 2005. [Google Scholar]
- 14.Rajpal V. Standerdization of Botanicals. Vol. 1. New Delhi: Eastern Publishers; 2002. pp. 39–44. [Google Scholar]


