Abstract
Background
Folic acid, vitamin B6, and vitamin B12 act in concert in the one-carbon metabolism and may protect against colorectal neoplasia. We examined the effect of combined B-vitamin treatment on the occurrence of colorectal adenoma.
Methods
The Women’s Antioxidant and Folic Acid Cardiovascular Study was a randomized, double-blind, placebo-controlled trial of 5442 female health professionals at high risk for cardiovascular disease from April 1998 through July 2005. Participants were randomly assigned to receive a combination pill of folic acid (2.5mg), vitamin B6 (50mg), and vitamin B12 (1mg) or placebo. This study included 1470 participants who were followed up for as long as 9.2 years and underwent an endoscopy at any point during follow-up. We estimated relative risks using a generalized linear model with a natural logarithm link function and Poisson distributed errors. All statistical tests were two-sided.
Results
The risk of colorectal adenoma was similar among participants receiving treatment (24.3%, 180 of 741 participants) vs placebo (24.0%, 175 of 729 participants) (multivariable adjusted relative risk = 1.00, 95% confidence interval = 0.83 to 1.20). Treatment was not associated with the risk of adenoma when data were analyzed by subsite, size, stage, and the number of adenomas. There was no statistically significant effect modification by alcohol intake, history of cancer or adenoma, or baseline plasma levels or intakes of folate, vitamin B6, or vitamin B12.
Conclusion
Our results indicate no statistically significant effect of combined folic acid, vitamin B6, and vitamin B12 treatment on colorectal adenoma among women at high risk for cardiovascular disease.
Folate, vitamin B6, and vitamin B12 are essential cofactors that play important roles in one-carbon metabolism, which is required for the maintenance of intracellular DNA synthesis and methylation. Data from both in vitro and animal studies have suggested a protective effect of B vitamins against colorectal carcinogenesis (1,2), although the results have been somewhat mixed and the complex mechanisms have not yet been fully elucidated. Observational evidence, though not entirely consistent, has suggested that blood levels of folate or vitamin B6 are inversely related to the risk of colorectal neoplasia. Some, but not all prospective studies have suggested a 20%–40% reduction in the risk of colorectal cancer or adenoma in those with the highest intake of folate compared with those with the lowest intake (1–3). A recent meta-analysis of nine prospective studies showed a 10% reduction in colorectal cancer in individuals with the highest vitamin B6 intake compared with those with the lowest intake (4).
However, most randomized trials have focused on folic acid supplementation alone and found neither beneficial nor harmful effects of folic acid supplementation (0.5–5mg/d for as long as 3 years) on recurrence of colorectal adenomas (1,2,5,6). Mandatory fortification of cereals and grain products with folic acid in the United States beginning in the year 1998 has improved folate status in the general population (7,8), but there has been concern about limited protective effects or a potential cancer-promoting effect of folic acid treatment alone in a folic acid–fortified population. Because folic acid, vitamin B6, and vitamin B12 act in concert to affect the pathways of one-carbon metabolism (1,2), combined folic acid, vitamin B6, and vitamin B12 supplements may have greater promise for preventing colorectal adenoma, the precursor of invasive cancer, than any single B-vitamin supplement. Combined B-vitamin supplementation also helps address the concern about potentially masking vitamin B12 deficiency by folic acid supplementation alone in adults. Because 28%–35% of the US population report the use of dietary supplements that contain folic acid and vitamins B6 and B12 (9,10), definitive data from large randomized trials with long treatment duration are warranted to evaluate the potential yet unproven benefits or risks of combined folic acid, vitamin B6, and vitamin B12 supplementation.
In the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), a large cardiovascular disease (CVD) prevention trial conducted during the folic acid fortification era, we specifically examined the effect of daily supplementation with folic acid, vitamin B6, and vitamin B12 on the occurrence of colorectal adenoma among participants followed up for as long as 9.2 years (7.3 years of active treatment and 1.9 years of postintervention follow-up).
Methods
Study Design and Population
The WAFACS was a randomized, double-blind, placebo-controlled trial evaluating the effects of combined folic acid, vitamin B6, and vitamin B12 in the secondary prevention of CVD among 5442 high-risk women with either a history of CVD or at least three cardiovascular risk factors (http://clinicaltrials.gov, identifier: NCT00000541) (11–14). The trial was approved by the institutional review board of Brigham and Women’s Hospital, and all participants provided written informed consent. An external independent data and safety monitoring board monitored the safety of the participants and the overall quality and scientific integrity of the trial.
Details of the WAFACS trial design and the main results have been reported previously (11–14). Briefly, from June 1995 through October 1996, 8171 female health professionals in the Women’s Antioxidant Cardiovascular Study (the parent trial of the WAFACS) were randomly assigned to receive vitamin C (500mg/d), vitamin E (600 IU every other day), and β-carotene (50mg every other day) vs respective matching placebos (11–14). Women were eligible for the Women’s Antioxidant Cardiovascular Study if they were at least 40 years of age, were postmenopausal, or had no intention of becoming pregnant, and had a self-reported history of CVD or had at least three traditional cardiac risk factors (12).
In April 1998, 5442 of these women provided consent and were additionally randomly assigned in a factorial design to receive a daily combination pill containing folic acid (2.5mg), vitamin B6 (pyridoxine hydrochloride, 50mg), and vitamin B12 (cyanocobalamin, 1mg) or a matching placebo to form the WAFACS trial population (11,12,14). Our study included 1470 WAFACS participants who underwent at least one endoscopy after random assignment to treatment and were followed up through June 30, 2007 (Figure 1).
Figure 1.
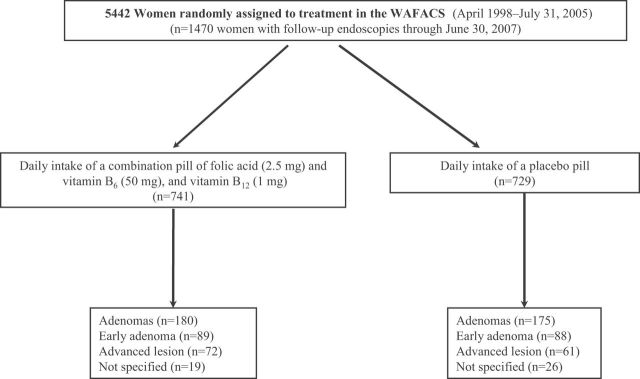
Flow diagram illustrating adenoma outcomes in the combination folic acid, vitamin B6, and vitamin B12 component of the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS). Early adenomas were defined as small and tubular. Advanced adenomas included large, tubular-villous, and villous adenomas, high-grade dysplasia, and colorectal cancer.
Follow-up Procedures
Following random assignment and annually thereafter, participants were mailed monthly calendar packs containing active agents or placebos, along with questionnaires on adherence, use of nonstudy supplements, and occurrence of major illnesses or adverse events. At baseline, 98.8% of the participants also answered a semiquantitative food-frequency questionnaire to assess dietary nutrient intakes (11–14). At the scheduled end of the trial (July 31, 2005), morbidity and mortality follow-up was 92.6% complete. If assessed in terms of person-time, mortality and morbidity information was complete for 98.9% and 98.0% of person-years of follow-up, respectively (11,13,14). The average adherence, defined as taking at least two-thirds of the study pills over the course of follow-up, was 83% for both the active and placebo groups.
Women in the WAFACS provided a baseline blood sample in the year 1996 (75.4% of the participants), before folic acid fortification in the year 1998 (7,8). Randomly selected from participants who were adherent with study medications, 300 (150 women in the active treatment group and 150 women in the placebo group) provided a blood sample at the end of randomized treatment. As reported previously (11,14), median plasma folate (8.8 vs 8.9ng/mL, P = .94) and homocysteine levels (12.1 vs 12.5 µmol/L, P = .96) were similar between the active treatment group and the placebo group at baseline. At the end of trial, the median plasma folate level in the active group was statistically significantly higher (38.9 vs 15.4ng/mL, P < .001) and homocysteine was statistically significantly lower (9.8 vs 11.8 µmol/L, P < .001) compared with the placebo group.
Ascertainment of Colorectal Adenoma
Participants in WAFACS who reported a recent endoscopy (colonoscopy and/or sigmoidoscopy) or a diagnosis of colon polyps on the trial questionnaires were mailed a medical release form to get permission to obtain and review relevant medical records from the treating physician and/or hospital. An endpoints committee of physicians who were blinded to the participants’ treatment assignment reviewed the medical records that included endoscopy and histopathologic reports for final confirmation of a reported colorectal adenoma diagnosis and/or endoscopy procedure through June 30, 2007. Additional details of polyp characteristics and indications for endoscopy procedures were also extracted from medical records. Colorectal polyps were first categorized by types (adenomatous, hyperplastic) and anatomic location within the large bowel. For adenomatous polyps, polyp characteristics were captured by the number, size, histologic type (tubular, tubulovillous, villous), and grade of dysplasia (low, high). Advanced histology neoplasms were defined as tubulovillous or villous adenomas, high-grade dysplasia (carcinoma in situ), or invasive cancer (15). Polyps were also classified into small (<1cm in diameter) and large (≥1cm). An advanced adenoma was defined as a lesion that had either a large size (≥1cm) or advanced histology. A total of 1470 participants who received at least one endoscopy during April 1998 through June 30, 2007 were included in this analysis, and of them, 355 had confirmed colorectal adenomas.
Laboratory Analysis
In this study, baseline plasma levels of folate, vitamin B6, vitamin B12, homocysteine, cysteine, and cysteinylglycine were assayed at the Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University. Plasma folate and vitamin B12 were determined by the IMMULITE 1000 immunoassay system (Diagnostic Products Corporation, Los Angeles, CA). Plasma levels of vitamin B6 were determined by an enzymatic procedure using radioactive tyrosine and the apoenzyme tyrosine decarboxylase (16). Plasma homocysteine, cysteine, and cysteinylglycine were measured using high-performance liquid chromatography, with fluorescence detection (17). The mean coefficients of variation for 22 replicate quality control plasma samples were 7.5% for folate, 15.9% for vitamin B6, 6.2% for vitamin B12, 8.3% for homocysteine, 9.7% for cysteine, and 9.0% for cysteinylglycine.
Statistical Analysis
Baseline characteristics were compared using Wilcoxon rank-sum test for continuous variables and Mantel–Haenszel χ2 statistics for categorical variables between the active treatment and placebo groups. At each 2-year follow-up interval, the point prevalence was calculated for all adenoma and advanced lesions by randomized treatment assignment, and the prevalence difference between two groups overall and at each 2-year follow-up interval was compared by the χ2 test. To further assess the effect of combined B-vitamin supplementation on the risk of adenoma, we estimated relative risks (RRs) by using a generalized linear model with a natural logarithm link function and Poisson distributed errors, which were adjusted for over- and underdispersion with SAS PROC GENMOD (SAS 9.1.2; SAS Institute Inc, Cary, NC). All statistical tests were two-sided. The “basic model” included age at start of trial (continuous) and time between start of trial and last follow-up endoscopy (months). The “full multivariable model” additionally included body mass index (continuous); smoking status (current, past, or never); alcohol intake (continuous); physical activity (continuous); menopausal status and postmenopausal hormone (PMH) use (premenopausal, uncertain, postmenopausal and current PMH use, postmenopausal and past PMH use, or postmenopausal and never use of PMH); total dietary and supplemental intakes of folate, vitamin B6, and vitamin B12 (all as continuous variables); history of cancer (yes or no); history of colorectal adenoma before random assignment (no adenoma, early adenoma, advanced adenoma, missing, or no endoscopy before random assignment); and randomized assignments of vitamin E (yes or no), vitamin C (yes or no), and β-carotene (yes or no). Also, we conducted analyses according to secondary outcome measures—ie, adenoma subsite (proximal or distal), size (small [<1 cm] or large [≥1 cm]), stage (early [small and tubular] or advanced [large or advanced histology]), and the number of adenomas (1 or ≥2 for the first endoscopy only).
Subgroup analyses were conducted to examine the effect of active treatment on risk of any colorectal adenoma according to prespecified baseline characteristics, including categories of age; body mass index; smoking; alcohol use; physical activity; menopausal status; PMH use; baseline total intakes of folate, vitamin B6, or vitamin B12; baseline plasma levels of folate, vitamin B6, vitamin B12; homocysteine, cysteine, or cysteinylglycine; current multivitamin use; CVD health history; history of cancer within the past 10 years; history of diabetes; history of endoscopy before random assignment; history of any adenoma before random assignment; randomization status for vitamin E, vitamin C, and β-carotene; and family history of colorectal cancer. Given a priori evidence of an interaction between folate and alcohol, we also conducted a stratified analysis by combined baseline plasma (> and ≤10.6ng/mL) or intake (> and ≤458.3 µg/d) levels of folate and alcohol status (0g/d and >0g/d). Log likelihood ratio tests comparing models with or without interaction terms were used to assess effect modifications between randomized combined B-vitamin treatment assignment and categories of baseline characteristics in relation to any adenoma risk.
Also, we conducted several sensitivity analyses to evaluate the robustness of the results. First, our participants were not screened for colorectal adenomas by colonoscopy before random assignment; thus, there is a possibility that some adenomas diagnosed during the intervention and follow-up period may have been prevalent at baseline. To address this, we conducted analyses excluding participants with colorectal adenomas that occurred within 2 years after the start of the trial. Second, to assess whether associations differed by indications for endoscopy (for screening or for symptoms), we also analyzed data among women who had an endoscopy for routine screening or who had it because of symptoms, separately (for the first endoscopy only). Third, to examine the effect of actual as opposed to assigned folic acid, vitamin B6, and vitamin B12 treatment, we carried out an analysis according to compliance with assigned treatment. Women were censored if and when they stopped taking at least two-thirds of their study pills or were missing compliance information.
On the basis of the actual numbers of our study population (n = 1470) and participants with colorectal adenomas detected on all endoscopies during follow-up (n = 355), we calculated the power of our main analysis by using a Stata program developed by Barthel et al. (18). The power computation in this program is based on the χ2 statistic with a continuity correction (18). All power calculations were performed using Intercooled Stata version 8.2 (Stata Corporation, College Station, TX). By assuming a two-sided α level at .05, we calculated power over a range of relative risks by one single-agent effect from 0.90 to 0.60 (ie, approximately 10%–30% reduction in risk). Using the numbers of study participants (n = 1470), participants with colorectal adenomas in the study population (n = 355), and those with colorectal adenomas in the placebo group (n = 175), we should have adequate power (≥80%) to detect more than a 22% reduction in the risk of total colorectal adenomas. Because of the smaller number of participants with advanced adenomas (n = 133), we had at least 80% power to detect a 37% or greater reduction in the risk of advanced adenomas.
Results
Characteristics of the Study Participants
Among the 1470 women who had at least one endoscopic follow-up after random assignment, 741 were allocated to active treatment and 729 to placebo, and there were no statistically significant differences in baseline characteristics between these two groups, including age (62.1 vs 61.8 years, respectively, P = .54), history of endoscopy before random assignment (16.1% vs 16.5%, respectively, P = .83), and the number of having more than one endoscopy during follow-up (34.3% vs 36.4%, respectively, P = .60)—ie, factors that may have affected the likelihood of a follow-up endoscopy (Table 1). Moreover, baseline characteristics of participants who were included in this study (n = 1470) were similar to the rest of WAFACS participants (n = 3972) (data not shown).
Table 1.
Baseline characteristics of women with follow-up endoscopies by treatment assignment with combined folic acid, vitamin B6, vitamin B12 treatment vs placebo in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS)
| Characteristics | Folic acid, vitamin B6, and vitamin B12 (n=741) | Placebo (n=729) | P* |
|---|---|---|---|
| Mean age, y (SD) | 62.1 (7.6) | 61.8 (7.6) | .54 |
| Mean body mass index (SD), kg/m2 | 30.6 (6.4) | 30.1 (6.0) | .09 |
| Smoking status, no. (%) | |||
| Current | 56 (7.6) | 55 (7.5) | .47 |
| Past | 328 (44.3) | 340 (46.6) | |
| Never | 357 (48.2) | 334 (45.8) | |
| Mean alcohol intake (SD), g/d | 3.2 (7.5) | 3.6 (7.7) | .16 |
| Mean physical activity, kcal/wk, no. (%) | |||
| <1000 | 445 (60.1) | 437 (59.9) | .97 |
| ≥1000 | 296 (39.9) | 292 (40.1) | |
| Menopausal status, no. (%) | |||
| Premenopausal | 41 (5.5) | 46 (6.3) | .17 |
| Uncertain | 23 (3.1) | 10 (1.4) | |
| Postmenopausal | 677 (91.4) | 673 (92.3) | |
| Postmenopausal hormone use, no. among postmenopausal women only (%) | |||
| Current | 426 (62.9) | 434 (64.5) | .59 |
| Past | 112 (16.5) | 107 (15.9) | |
| Never | 139 (20.5) | 132 (19.6) | |
| Baseline dietary intake | |||
| Total folate, mean (SD) µg† | 516.3 (259.6) | 515.0 (234.5) | .66 |
| Total vitamin B6, mean (SD), mg† | 5.1 (15.2) | 5.6 (17.4) | .99 |
| Total vitamin B12, mean (SD), µg† | 9.3 (11.4) | 9.5 (9.5) | .54 |
| Red meat (servings/d) | 0.8 (0.6) | 0.8 (0.6) | .20 |
| Processed meat (servings/d) | 0.1 (0.2) | 0.1 (0.2) | .82 |
| Methionine, mean (SD), g/d† | 1.9 (0.6) | 2.0 (0.7) | .65 |
| Baseline plasma concentrations | |||
| Folate, mean (SD), ng/mL | 13.1 (8.3) | 13.9 (8.9) | .29 |
| Vitamin B6, mean (SD), pmol/mL | 65.8 (58.7) | 61.3 (50.5) | .24 |
| Vitamin B12, mean (SD), pg/mL | 490.5 (227.7) | 494.2 (216.9) | .48 |
| Homocysteine, mean (SD), nmol/mL | 11.0 (4.2) | 11.1 (4.1) | .89 |
| Cysteine, mean (SD), nmol/mL | 277.8 (49.2) | 274.9 (49.9) | .37 |
| Cysteinylglycine, mean (SD), nmol/mL | 345.6 (184.6) | 346.6 (150.6) | .59 |
| Current multivitamin use before random assignment, no. (%) | |||
| Yes | 188 (25.4) | 176 (24.1) | .59 |
| No | 553 (74.6) | 553 (75.9) | |
| CVD health history, no. (%)‡ | |||
| Previous CVD | 461 (62.2) | 440 (60.4) | .47 |
| ≥Three risk factors | 280 (37.8) | 289 (39.6) | |
| History of cancer within past 10 years except nonmelanoma skin cancers, no. (%) | |||
| Yes | 38 (5.1) | 50 (6.9) | .16 |
| No | 703 (94.9) | 679 (93.1) | |
| History of diabetes, no. (%) | |||
| Yes | 120 (16.2) | 94 (12.9) | .07 |
| No | 621 (83.8) | 635 (87.1) | |
| History of endoscopy before random assignment, no. (%) | |||
| Yes | 119 (16.1) | 120 (16.5) | .83 |
| No | 622 (83.9) | 609 (83.5) | |
| History of any adenoma before random assignment among those with prerandomization endoscopy, no. (%) among those with prerandomization endoscopy | |||
| No adenoma | 77 (64.7) | 91 (75.8) | .43 |
| Early adenoma (small/tubular) | 20 (16.8) | 8 (6.7) | |
| Advanced adenoma (large or villous histology or high-grade dysplasia) | 15 (12.6) | 11 (9.2) | |
| Stage not specified/missing | 7(5.9) | 10 (8.3) | |
| No. of adenomas before random assignment among those with prerandomization endoscopy (%) | |||
| 0 | 77 (64.7) | 91 (75.8) | .09 |
| 1 | 26 (21.9) | 18 (15.0) | |
| 2 | 9 (7.6) | 7 (5.8) | |
| ≥3 | 7 (5.9) | 4 (3.3) | |
| Total no. of endoscopies during follow-up (%)§ | |||
| 1 | 487 (65.7) | 464 (63.7) | .60 |
| 2 | 182 (24.6) | 196 (26.9) | |
| ≥3 | 72 (9.7) | 69 (9.5) | |
| Time between start of trial and endoscopy (mo)§ | |||
| First endoscopy | 52.2 (28.4) | 52.7 (27.9) | .84 |
| Second endoscopy | 75.8 (22.7) | 75.0 (22.5) | .70 |
| Last endoscopy | 71.9 (25.3) | 72.8 (24.5) | .58 |
| No. randomly assigned to receive vitamin E (%) | |||
| Yes | 364 (49.1) | 373 (51.2) | .43 |
| No | 377 (50.9) | 356 (48.8) | |
| No. randomly assigned to receive vitamin C (%) | |||
| Yes | 390 (52.6) | 369 (50.6) | .44 |
| No | 351 (47.4) | 360 (49.4) | |
| No. randomly assigned to receive β-carotene (%) | |||
| Yes | 374 (50.5) | 358 (49.1) | .60 |
| No | 367 (49.5) | 371 (50.9) | |
* Two-sided P values were calculated by using Wilcoxon rank-sum test for continuous variables and Mantel–Haenszel χ2 test for categorical variables. CVD = cardiovascular disease; PMH = postmenopausal hormone.
† The energy-adjusted nutrient intake was calculated.
‡ CVD health history was categorized as women with a self-reported history of myocardial infarction, stroke, coronary revascularization, angina pectoris, transient ischemic attack, carotid endarterectomy, or peripheral artery surgery; or those with no previous CVD but with at least three of the following: hypertension, high cholesterol level, diabetes mellitus, parental history of premature myocardial infarction (before age 60 years), obesity (body mass index ≥30kg/m2), current cigarette smoking, and inconsistent report of previous CVD.
§ Endoscopies performed between May 1, 1998 (the initiation of trial) and June 30, 2007 (≤23 months after completion of the trial) (trial ended on July 31, 2005) were counted as endoscopies performed during the study period.
Most adenomas that were detected were small, early, proximal, and tubular (Table 2). The proportions of participants with two or more adenomas were identical in the active treatment group and in the placebo group (38.9% for both groups, P > .99). The proportions of women with large adenomas were also similarly distributed in the active treatment group and in the placebo group (30.6% vs 30.9%, respectively, P = .94). There were similar frequencies of participants with advanced lesions between women randomly assigned to receive combined B-vitamin treatment vs placebo (40.0% vs 34.9%, respectively, P = .64).
Table 2.
Adenoma characteristics in participants with follow-up endoscopies by treatment assignment with combination folic acid, vitamin B, and vitamin B12 treatment vs placebo in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS)
| Adenoma characteristics | Folic acid, vitamin B6, and vitamin B12 (n=180) | Placebo (n=175) | P* |
|---|---|---|---|
| Location of any adenoma, no. (%) | |||
| Rectum only | 22 (12.2) | 15 (8.6) | .07 |
| Distal only | 53 (29.4) | 41 (23.4) | |
| Proximal only | 73 (40.6) | 81 (46.3) | |
| ≥2 locations | 30 (16.7) | 35 (20.0) | |
| Not specified | 2 (1.1) | 3 (1.7) | |
| Histologic type of adenoma†, no. (%) | |||
| Tubular | 98 (54.4) | 92 (52.6) | .48 |
| Tubular-villous | 26 (14.4) | 19 (10.9) | |
| Villous | 2 (1.1) | 5 (2.9) | |
| Mixed | 14 (7.8) | 15 (8.6) | |
| High-grade dysplasia/colorectal cancer | 15 (8.3) | 13 (7.4) | |
| Not specified | 25 (13.9) | 31 (17.7) | |
| Size of adenoma (largest size detected), no. (%) | |||
| Small | 122 (67.8) | 118 (67.4) | .94 |
| Large (≥10mm) | 55 (30.6) | 54 (30.9) | |
| Not specified | 3 (1.7) | 3 (1.7) | |
| Stage of adenoma (worst stage diagnosed), no. (%) | |||
| Early (small and tubular) | 89 (49.4) | 88 (50.3) | .64 |
| Advanced (large or tubular-villous or villous or high-grade dysplasia or colorectal cancer) | 72 (40.0) | 61 (34.9) | |
| Not specified | 19 (10.6) | 26 (14.9) | |
| No. of adenomas (%)‡ | |||
| 1 | 110 (61.1) | 107 (61.1) | >.99 |
| ≥2 | 70 (38.9) | 68 (38.9) | |
| Mean time between start of trial and first adenoma, mo (SD)§ | 61.1 (28.3) | 57.1 (29.5) | .22 |
* Two-sided P values were calculated by using Mantel–Haenszel χ2 test.
† Worst histology of any adenoma.
‡ The sum of all adenomas diagnosed during the follow-up period regardless of the frequency of endoscopy.
§ The difference between the means was 4.01 (95% confidence interval [CI] = −2.03 to 10.04).
Total Colorectal Adenoma and Subtypes
Figure 2A shows the point prevalence of all adenoma events among women in the treatment and placebo groups at every 2-year follow-up period after random assignment. There appeared to be a trend toward a higher prevalence in the active group compared with the placebo group, but this difference did not achieve statistical significance. There was no difference in the point prevalence of all advanced lesions between the two groups overall and at each 2-year follow-up period (all P > .05) (Figure 2B).
Figure 2.
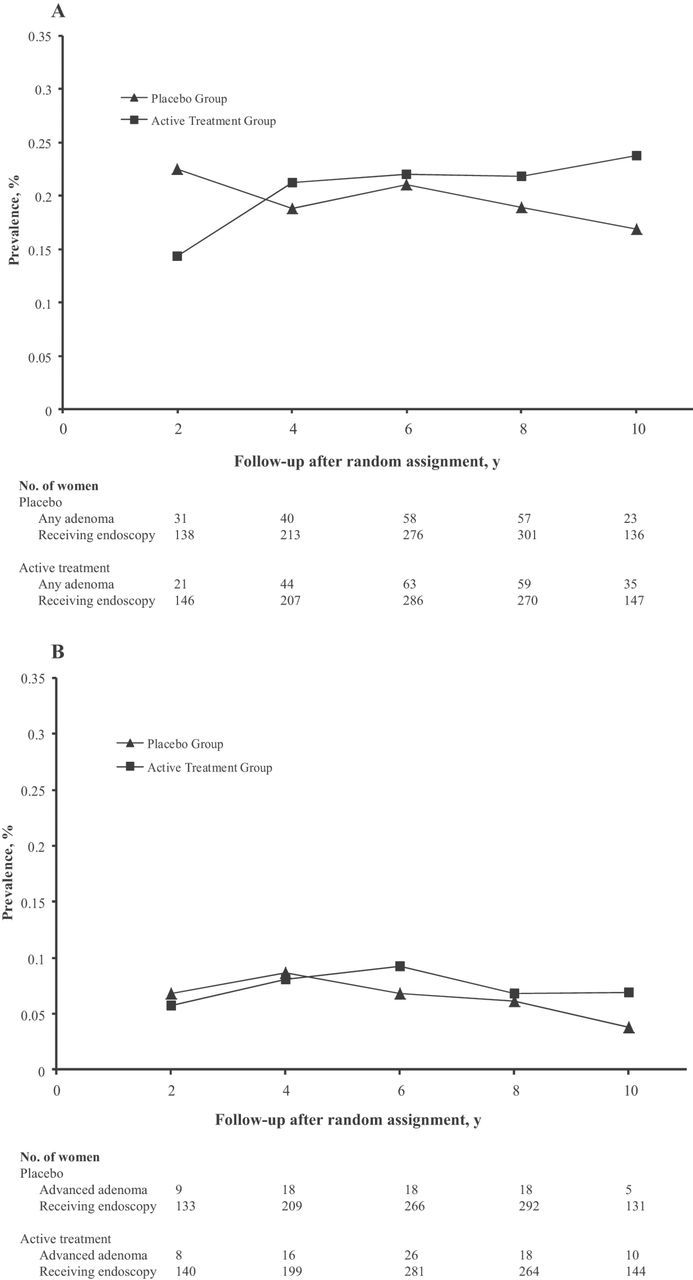
Prevalence of any colorectal adenoma and advanced colorectal lesions among participants in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS). Women were grouped by random assignment to combined folic acid, vitamin B6, and vitamin B12 (active treatment) or placebo. The prevalence of (A) any colorectal adenoma and (B) advanced colorectal lesions among the two treatment groups is shown. The number of women in each group at the time of follow-up is given in a table below the figure.
Overall, there was no effect of combined folic acid, vitamin B6, and vitamin B12 treatment on the risk of colorectal adenoma (Table 3). There were 180 participants with incident adenomas (24.3%) in the active treatment group (n = 741) and 175 (24.0%) in the placebo group (n = 729), corresponding to an overall relative risk of 1.02 (95% confidence interval [CI] = 0.85 to 1.23) after controlling for age and time between the start of the trial and the last endoscopy. The results were unchanged after further adjustment for other colorectal cancer risk factors (RR = 1.00, 95% CI = 0.83 to 1.20). When we classified all adenomas by the adenoma subsite (proximal or distant colon), size (small or large), stage (early or advanced), and the number of adenomas at the first endoscopy after random assignment, we found no indication of a statistically significant difference for any of these outcomes (Table 3).
Table 3.
Relative Risks (RRs) of adenoma by treatment assignment with combination folic acid, vitamin B, vitamin B12 (active) treatment vs placebo in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS)*
| First follow-up endoscopy | All follow-up endoscopies (first–last) | |||||||
|---|---|---|---|---|---|---|---|---|
| Active (n=741) | Placebo (n=729) | RR (95% CI)† | RR (95% CI)‡ | Active (n=741) | Placebo (n=729) | RR (95% CI)§ | RR (95% CI)‖ | |
| Outcome | No. of individuals with adenomas/total participants | No. of individuals with adenomas/total participants | No. of individuals with adenomas/total participants | No. of individuals with adenomas/total participants | ||||
| At least one adenoma | 145/741 | 141/729 | 1.01 (0.82 to 1.24) | 0.97 (0.78 to 1.20) | 180/741 | 175/729 | 1.02 (0.85 to 1.23) | 1.00 (0.83 to 1.20) |
| Subsite of adenoma¶ | ||||||||
| ≥1 proximal adenoma§ | 68/740 | 79/727 | 0.84 (0.62 to 1.15) | 0.83 (0.61 to 1.14) | 101/739 | 110/726 | 0.91 (0.71 to 1.17) | 0.90 (0.70 to 1.17) |
| ≥1 distal adenoma | 66/740 | 54/727 | 1.20 (0.85 to 1.69) | 1.10 (0.77 to 1.57) | 79/739 | 71/726 | 1.10 (0.81 to 1.49) | 0.98 (0.71 to 1.34) |
| Size of adenoma¶ | ||||||||
| Small | 101/738 | 96/726 | 1.03 (0.80 to 1.34) | 1.00 (0.77 to 1.31) | 122/738 | 118/726 | 1.03 (0.82 to 1.30) | 1.02 (0.80 to 1.29) |
| Large | 41/738 | 42/726 | 0.96 (0.63 to 1.45) | 0.91 (0.59 to 1.40) | 55/738 | 54/726 | 1.01 (0.71 to 1.45) | 0.97 (0.67 to 1.40) |
| Stage of adenoma¶ | ||||||||
| Early | 74/724 | 71/708 | 1.02 (0.75 to 1.39) | 0.98 (0.71 to 1.34) | 89/722 | 88/703 | 0.99 (0.75 to 1.31) | 0.98 (0.74 to 1.30) |
| Advanced | 54/724 | 49/708 | 1.07 (0.74 to 1.56) | 1.03 (0.70 to 1.51) | 72/722 | 61/703 | 1.16 (0.83 to 1.60) | 1.06 (0.76 to 1.50) |
| No. of adenomas (first endoscopy only) | ||||||||
| 1 | 102/741 | 102/729 | 0.99 (0.76 to 1.27) | 0.97 (0.74 to 1.26) | NA | NA | NA | NA |
| ≥2 | 43/741 | 39/729 | 1.08 (0.71 to 1.64) | 0.93 (0.61 to 1.43) | NA | NA | NA | NA |
* The generalized linear model with a natural logarithm link function and Poisson distributed errors were used. CI = confidence interval; NA = not available; PMH = postmenopausal hormone.
† Basic models included the age at the start of trial (continuous) and the time between the start of trial and first endoscopy (months).
‡ Full models included the age at the start of trial (continuous), the time between start of trial and first endoscopy (months), body mass index (continuous), smoking status (current, past, or never), alcohol intake (continuous), physical activity (continuous), menopausal status and PMH use (premenopausal, uncertain, postmenopausal and current PMH use, postmenopausal and past PMH use, or postmenopausal and never use of PMH), baseline folate intake (continuous), baseline vitamin B6 intake (continuous), baseline vitamin B12 intake (continuous), history of cancer (yes or no), history of adenoma before random assignment (no adenoma, early adenoma, advanced adenoma, missing, or no endoscopy before random assignment), randomly assigned to receive vitamin E (yes or no), randomly assigned to receive vitamin C (yes or no), and randomly assigned to receive β-carotene (yes or no).
§ Basic models included age at start of trial (continuous) and time between start of the trial and last endoscopy (months).
‖ Full models are the same as those for all first follow-up endoscopies except that the time between the start of trial and last endoscopy (months) was used.
¶ The denominator does not include participants with missing or unknown information for site, size, or stage. It does include those with no adenoma for site, size, and stage.
Subgroup Analysis
The effect of B-vitamin supplementation on adenoma risk did not statistically significantly differ by age, body mass index, smoking, alcohol use, physical activity, menopausal status, PMH use, or baseline intakes of folate, vitamin B6, or vitamin B12 (Table 4). The results were also similar by baseline plasma levels of folate, vitamin B6, vitamin B12, homocysteine, cysteine, or cysteinylglycine. Furthermore, the relative risks were similar as well by current multivitamin use, CVD health history, history of cancer within the past 10 years, history of diabetes, history of endoscopy before random assignment, and history of any adenoma before random assignment. No statistically significant difference in the effect of B-vitamin supplementation on adenoma risk was observed among the groups in terms of randomized assignment to vitamin E, vitamin C, or β-carotene or family history of colorectal cancer. The relative risks were also similar among women who had low alcohol intake and high plasma level or intake of folate and among those who had high alcohol intake and low plasma level or intake of folate (P interaction ≥.64). Combined B-vitamin treatment statistically significantly reduced the risk of colorectal adenoma in women with a history of adenoma before random assignment in a multivariable analysis (RR = 0.48, 95% CI = 0.24 to 0.94), though the test for interaction was not statistically significant (P = .15). Although there was no evidence that any single covariate caused substantial changes in the estimates, simultaneous adjustment for smoking, folate intake, and vitamin C treatment resulted in the most statistically significant changes from the basic model (RR = 0.70, 95% CI = 0.42 to 1.16) to the full model (RR = 0.55, 95% CI = 0.32 to 0.93).
Table 4.
Relative risks (RR) of any adenoma by treatment assignment with combination folic acid, vitamin B6, vitamin B12 (active) treatment vs placebo, by baseline characteristics of participants in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS)*
| Active—no. of individuals with adenomas /total participants | Placebo—no. of individuals with adenomas /total participants | Basic adjusted model† | Multivariable-adjusted model‡ | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | RR (95% CI) | P | Pinteraction§ | RR (95% CI) | P | Pinteraction§ | ||
| Median age, y | ||||||||
| ≤62.3 | 80/365 | 87/370 | 0.94 (0.72 to 1.23) | .68 | .25 | 0.95 (0.71 to 1.25) | .69 | .53 |
| >62.3 | 100/376 | 88/359 | 1.10 (0.86 to 1.41) | .45 | 1.06 (0.82 to 1.38) | .64 | ||
| Body mass index, kg/m2 | ||||||||
| <25 | 34/154 | 34/155 | 1.04 (0.68 to 1.60) | .85 | .92 | 0.97 (0.60 to 1.57) | .89 | .69 |
| 25–29 | 48/211 | 52/219 | 0.95 (0.68 to 1.33) | .78 | 0.85 (0.58 to 1.23) | .38 | ||
| ≥30 | 98/376 | 89/355 | 1.05 (0.82 to 1.35) | .70 | 1.07 (0.82 to 1.39) | .62 | ||
| Smoking status | ||||||||
| Current | 19/56 | 21/55 | 0.96 (0.58 to 1.60) | .88 | .73 | 0.79 (0.43 to 1.44) | .44 | .47 |
| Past | 72/328 | 73/340 | 1.12 (0.84 to 1.49) | .43 | 1.13 (0.84 to 1.53) | .42 | ||
| Never | 89/357 | 81/334 | 0.96 (0.74 to 1.25) | .75 | 0.92 (0.70 to 1.22) | .57 | ||
| Alcohol intake, g/d | ||||||||
| 0 | 97/379 | 90/357 | 1.03 (0.81 to 1.33) | .79 | .99 | 0.99 (0.76 to 1.29) | .92 | .94 |
| 1–4 | 55/241 | 55/228 | 1.00 (0.72 to 1.39) | >.99 | 0.90 (0.63 to 1.28) | .54 | ||
| ≥5 | 28/121 | 30/144 | 0.94 (0.59 to 1.49) | .79 | 1.05 (0.65 to 1.72) | .83 | ||
| Physical activity, kcal/wk | ||||||||
| <1000 | 109/445 | 109/437 | 1.01 (0.82 to 1.24) | .94 | .82 | 0.96 (0.76 to 1.22) | .76 | .66 |
| ≥1000 | 71/296 | 66/292 | 1.06 (0.74 to 1.51) | .75 | 1.07 (0.78 to 1.47) | .67 | ||
| Menopausal status | ||||||||
| Premenopausal | 6/41 | 12/46 | 0.55 (0.24 to 1.29) | .17 | .14 | 0.74 (0.31 to 1.77) | .50 | .16 |
| Uncertain | 5/23 | 5/10 | 0.55 (0.21 to 1.47) | .23 | 0.17 (0.02 to 1.86) | .15 | ||
| Postmenopausal | 169/677 | 158/673 | 1.07 (0.89 to 1.30) | .46 | 1.05 (0.86 to 1.28) | .63 | ||
| PMH use among postmenopausal women only | ||||||||
| Current | 98/426 | 93/434 | 1.08 (0.84 to 1.39) | .54 | .82 | 1.06 (0.82 to 1.36) | .68 | .81 |
| Past | 30/112 | 32/107 | 0.95 (0.62 to 1.45) | .80 | 0.90 (0.56 to 1.44) | .65 | ||
| Never | 41/139 | 33/132 | 1.14 (0.77 to 1.70) | .51 | 1.12 (0.70 to 1.79) | .63 | ||
| Median total nutrient intake | ||||||||
| Folate, µg/d | ||||||||
| ≤458.3 | 94/366 | 85/337 | 1.03 (0.80 to 1.33) | .81 | .93 | 1.01 (0.79 to 1.31) | .92 | .81 |
| >458.3 | 80/350 | 81/353 | 1.01 (0.77 to 1.33) | .92 | 0.98 (0.74 to 1.30) | .88 | ||
| Vitamin B6, mg/d | ||||||||
| ≤2.6 | 96/362 | 90/341 | 0.98 (0.77 to 1.25) | .86 | .63 | 0.98 (0.76 to 1.25) | .84 | .66 |
| >2.6 | 78/354 | 76/349 | 1.07 (0.81 to 1.42) | .62 | 1.02 (0.76 to 1.36) | .89 | ||
| Vitamin B12, mg/d | ||||||||
| ≤7.2 | 96/363 | 86/341 | 1.03 (0.80 to 1.32) | .80 | .92 | 1.02 (0.79 to 1.32) | .87 | 1.00 |
| >7.2 | 78/353 | 80/349 | 1.01 (0.77 to 1.33) | .95 | 1.01 (0.76 to 1.34) | .96 | ||
| Median plasma levels | ||||||||
| Folate, ng/mL | ||||||||
| ≤10.6 | 74/269 | 64/265 | 1.10 (0.82 to 1.47) | .54 | .52 | 1.14 (0.83 to 1.56) | .41 | .41 |
| >10.6 | 60/259 | 64/274 | 0.94 (0.69 to 1.29) | .71 | 0.94 (0.67 to 1.32) | .72 | ||
| Vitamin B6, pmol/mL | ||||||||
| ≤47.4 | 68/250 | 64/262 | 1.10 (0.81 to 1.48) | .54 | .74 | 1.08 (0.79 to 1.49) | .62 | .57 |
| >47.4 | 64/255 | 60/257 | 1.01 (0.74 to 1.37) | .95 | 0.97 (0.69 to 1.36) | .86 | ||
| Vitamin B12, pg/mL | ||||||||
| ≤430.0 | 64/263 | 72/271 | 0.87 (0.65 to 1.17) | .35 | .10 | 0.89 (0.66 to 1.22) | .47 | .21 |
| >430.0 | 70/265 | 56/268 | 1.25 (0.91 to 1.70) | .16 | 1.21 (0.86 to 1.70) | .28 | ||
| Homocysteine, nmol/mL | ||||||||
| ≤10.4 | 66/267 | 61/267 | 1.05 (0.77 to 1.43) | .74 | .92 | 1.10 (0.79 to 1.52) | .59 | .69 |
| >10.4 | 69/262 | 67/272 | 1.02 (0.76 to 1.37) | .89 | 0.97 (0.81 to 1.32) | .82 | ||
| Cysteine, nmol/mL | ||||||||
| ≤272.1 | 61/262 | 60/273 | 1.02 (0.75 to 1.40) | .88 | .94 | 1.14 (0.81 to 1.59) | .46 | .92 |
| >272.1 | 74/267 | 68/266 | 1.05 (0.79 to 1.39) | .75 | 1.03 (0.76 to 1.39) | .86 | ||
| Cysteinylglycine, nmol/mL | ||||||||
| ≤315.8 | 62/273 | 57/261 | 1.00 (0.73 to 1.38) | .99 | .77 | 1.06 (0.75 to 1.49) | .75 | .78 |
| >315.8 | 73/256 | 71/278 | 1.07 (0.81 to 1.42) | .63 | 1.04 (0.78 to 1.40) | .78 | ||
| Combination of alcohol/total folate intake at baseline | ||||||||
| 0g/d and >458.3 µg/d | 45/176 | 39/165 | 1.12 (0.76 to 1.63) | .57 | .65 | 1.01 (0.67 to 1.53) | .97 | .64 |
| >0g/d and ≤458.3 µg/d | 45/176 | 39/163 | 1.11 (0.76 to 1.63) | .58 | 1.06 (0.72 to 1.56) | .76 | ||
| Combination of alcohol/plasma folate at baseline | ||||||||
| 0g/d and >10.6ng/mL | 32/124 | 40/139 | 0.91 (0.61 to 1.36) | .65 | .64 | 0.97 (0.61 to 1.54) | .89 | .85 |
| >0g/d and ≤10.6ng/mL | 35/134 | 37/141 | 0.97 (0.65 to 1.45) | .87 | 1.03 (0.67 to 1.59) | .88 | ||
| Current multivitamin use | ||||||||
| Yes | 43/188 | 43/176 | 0.93 (0.64 to 1.35) | .70 | .56 | 0.67 (0.43 to 1.04) | .07 | .12 |
| No | 137/553 | 132/553 | 1.06 (0.86 to 1.30) | .61 | 1.08 (0.87 to 1.34) | .50 | ||
| CVD health history | ||||||||
| Previous CVD | 118/461 | 103/440 | 1.11 (0.88 to 1.39) | .39 | .30 | 1.06 (0.83 to 1.35) | .63 | .38 |
| ≥3 risk factors | 62/280 | 72/289 | 0.90 (0.67 to 1.22) | .51 | 0.94 (0.68 to 1.31) | .74 | ||
| History of cancer | ||||||||
| Yes | 9/38 | 13/50 | 1.13 (0.52 to 2.42) | .76 | .70 | 0.95 (0.37 to 2.42) | .91 | .87 |
| No | 171/703 | 162/679 | 1.01 (0.84 to 1.22) | .88 | 0.99 (0.82 to 1.21) | .94 | ||
| History of diabetes | ||||||||
| Yes | 31/120 | 19/94 | 1.32 (0.80 to 2.17) | .27 | .27 | 1.11 (0.64 to 1.91) | .72 | .26 |
| No | 149/621 | 156/635 | 0.98 (0.81 to 1.20) | .86 | 0.97 (0.79 to 1.19) | .77 | ||
| History of endoscopy before random assignment | ||||||||
| Yes | 32/119 | 32/120 | 1.01 (0.66 to 1.54) | .97 | >.99 | 0.84 (0.51 to 1.38) | .49 | .84 |
| No | 148/622 | 143/609 | 1.03 (0.84 to 1.25) | .81 | 1.02 (0.83 to 1.25) | .85 | ||
| History of any adenoma before random assignment‖ | ||||||||
| Yes | 18/42 | 16/29 | 0.70 (0.42 to 1.16) | .17 | .22 | 0.48 (0.24 to 0.94) | .03 | .15 |
| No | 14/77 | 16/91 | 1.18 (0.61 to 2.31) | .62 | 1.06 (0.52 to 2.17) | .88 | ||
| Randomly assigned to receive vitamin E | ||||||||
| Yes | 93/364 | 95/373 | 1.08 (0.84 to 1.37) | .56 | .61 | 1.05 (0.81 to 1.36) | .72 | .61 |
| No | 87/377 | 80/356 | 0.98 (0.75 to 1.28) | .88 | 0.94 (0.71 to 1.24) | .65 | ||
| Randomly assigned to receive vitamin C | ||||||||
| Yes | 98/390 | 79/369 | 1.17 (0.90 to 1.51) | .23 | .16 | 1.17 (0.89 to 1.53) | .27 | .08 |
| No | 82/351 | 96/360 | 0.90 (0.70 to 1.17) | .43 | 0.86 (0.65 to 1.12) | .26 | ||
| Randomly assigned to receive β-carotene | ||||||||
| Yes | 91/374 | 89/358 | 1.00 (0.78 to 1.29) | .99 | .80 | 0.91 (0.69 to 1.19) | .47 | .46 |
| No | 89/367 | 86/371 | 1.05 (0.81 to 1.36) | .72 | 1.11 (0.84 to 1.45) | .47 | ||
| Family history of colorectal cancer | ||||||||
| Yes | 39/134 | 41/150 | 1.11 (0.76 to 1.61) | .60 | .66 | 1.02 (0.69 to 1.51) | .93 | .86 |
| No | 137/597 | 131/574 | 1.00 (0.81 to 1.24) | .97 | 1.02 (0.82 to 1.27) | .87 | ||
* The generalized linear models with a natural logarithm link function and Poisson distributed errors were used. CI = confidence interval; CVD = cardiovascular disease; PMH = postmenopausal hormone.
† Basic models included the age at start of trial (continuous) and the time between start of trial and last endoscopy (months).
‡ Full models included the age at start of trial (continuous), the time between start of trial and last endoscopy (months), body mass index (continuous), smoking status (current, past, or never), alcohol intake (continuous), physical activity (continuous), menopausal status and PMH use (premenopausal, uncertain, postmenopausal and current PMH use, postmenopausal and past PMH use, or postmenopausal and never use of PMH), total folate intake (continuous), total vitamin B6 intake (continuous), total vitamin B12 intake (continuous), history of cancer (yes or no), history of adenoma before random assignment (no adenoma, early adenoma, advanced adenoma, missing, or no endoscopy before randomization), randomly assigned to receive vitamin E (yes or no), randomly assigned to receive vitamin C (yes or no), and randomly assigned to receive β-carotene (yes or no).
§ Pinteraction was assessed by comparing models with or without multiplicative interaction terms of randomized combined B-vitamin treatment assignment and categories of the stratified variable of interest using log likelihood ratio tests.
‖ Among those who underwent at least one endoscopy before random assignment.
Sensitivity Analysis
In sensitivity analyses, combined folic acid, vitamin B6, and vitamin B12 treatment was not statistically significantly associated with adenoma risk after excluding those with adenomas within the first 2 years of treatment and follow-up (RR = 1.07, 95% CI = 0.87 to 1.32, P = .53) or after restricting analysis to either participants who had endoscopy before randomization (RR = 1.01, 95% CI = 0.66 to 1.54, P = .97) or those who did not have endoscopy before random assignment (RR = 1.03, 95% CI = 0.84 to 1.25, P = .81). Also, there were no statistically significant effects on adenoma risk in the analysis by indications for the first endoscopy (RR = 1.16, 95% CI = 0.86 to 1.56, P = .34 for screening vs RR = 0.84, 95% CI = 0.63 to 1.34, P = .26 for symptoms). In a separate analysis in which women were censored at the time they stopped taking at least two-thirds of their study pills or had missing pill compliance, the relative risks of adenoma were also similar for any adenoma identified by the first endoscopy (RR = 0.98, 95% CI = 0.76 to 1.26, P = .86) and for any adenoma identified by all endoscopies during the follow-up (RR = 1.02, 95% CI = 0.81 to 1.29, P = .88).
Discussion
In the WAFACS trial, as much as 7.3 years of treatment (plus 1.9 years of postintervention follow-up) with combined folic acid, vitamin B6, and vitamin B12 did not have a statistically significant effect on the risk of colorectal adenoma among women who underwent at least one endoscopy during follow-up. We also found no statistically significant effect modifications by age; body mass index; smoking; alcohol intake; physical activity; menopausal status; PMH use; baseline plasma levels or intakes of folate, vitamins B6, and B12; current multivitamin use; and history of cancer, endoscopy, or adenoma. Our null findings for colorectal adenoma are consistent with our earlier report from the WAFACS (14), in which we found no effect of combined B-vitamin treatment on colorectal cancer risk.
Experimental data have suggested that B vitamins may help reduce the risk of colorectal cancer or adenoma via multiple metabolic pathways (1,2). In particular, vitamin B6 may prevent against colorectal neoplasia through several biological pathways other than one-carbon metabolism, including the suppression of cell proliferation, oxidative stress, nitric oxide synthesis, and angiogenesis (19). Observational epidemiologic studies, though not entirely consistent, tend to support the hypothesis that adequate intake of folate may reduce the risk of colorectal neoplasia (1–3). Observational evidence for an association between vitamin B6 and risk of colorectal neoplasia is also inconclusive despite several studies showing an inverse association between dietary vitamin B6 intake and blood vitamin B6 levels with colorectal cancer and colorectal adenoma (4). However, observational evidence is prone to some inherent biases, especially confounding from unmeasured or imperfectly measured factors and highly correlated dietary or lifestyle factors as well as measurement errors.
Data from randomized trials of combined B-vitamin supplementation for colorectal neoplasia have been limited. Most previous trials have focused on folic acid supplementation alone (1,2,5,6). A recent pooled analysis of data from three randomized trials found no statistically significant decrease or increase in the occurrence of new adenomas among 2632 participants who had a history of adenoma randomly assigned to either 0.5 or 1.0mg/d of folic acid or placebo and followed up for as long as 3.5 years (6). However, folic acid treatment was related to a non-statistically significantly reduced risk of any adenoma among participants in the lowest quartile of baseline plasma folate. The Aspirin/Folate Polyp Prevention Study found no evidence for overall risk reduction by folate supplementation (1mg/d) after 3 years of intervention but showed indications of an increased risk for advanced lesions and multiple adenomas after an additional 3–5 years of intervention (20). Our study used a much higher dose of folic acid (2.5mg/d) in combination with vitamin B6 and vitamin B12 for as long as 9.2 years and found that the effect on the occurrence of colorectal adenomas was similar compared with placebo, including large or advanced lesions, or even in those with low baseline plasma levels and intakes of B vitamins. Our null findings were consistent with two other large randomized trials of folic acid supplementation (15,21), a meta-analysis of five trials (5), and a pooled analysis of three large trials including the Aspirin/Folate Polyp Prevention Study (6). In contrast to findings in some observational studies of vitamin B6 (4), our results showed a null net effect of combined B-vitamin supplementation that contains vitamin B6 for the prevention of colorectal adenoma.
There has been considerable debate about the potential dual modulating roles of folate in colorectal cancer development and progression that were observed in animal studies and indicated in some human studies (2,20,22–24). Because mandatory folic acid fortification in the United States beginning in the year 1998 may reduce the prevalence of folate deficiency in the general population, it is critically important to assess the potential yet unproven benefits or risks of folic acid supplementation associated with the occurrence of colorectal adenomas, which are precursors of invasive cancer, in a folic acid–fortified population. Notably, it has been hypothesized that folic acid supplementation promotes colorectal carcinogenesis in susceptible individuals (ie, those with established colorectal neoplasms or with high folate intake) (2,20,22–24). Given the high dose of folic acid used in the WAFACS trial and the duration of the trial mostly in the folic acid fortification era, our study is better powered to test potential adverse effects of folic acid. However, our findings do not support the hypothesis that folic acid supplementation increases colorectal adenoma risk in susceptible individuals as we found no statistically significant effect of combined B-vitamin supplementation in the analyses restricted to participants with a history of cancer before random assignment, or with high baseline plasma levels or intakes of folate, or who were current users of multivitamin supplements at baseline.
Moreover, we found a statistically significant reduction in risk of adenoma among women with a history of adenoma before random assignment (a group that resembles the traditional study design for adenoma, which entails colonoscopy screening and adenoma removal at the time of enrollment). On the contrary, a pooled analysis of three large randomized trials among 2632 men and women (6) and a meta-analysis of five trials involving 1580 participants (5) did not show clear trends of decreased or increased occurrence of new adenomas associated with folic acid supplementation in participants with a history of adenoma. In our study, the numbers of participants who had a history of adenoma before random assignment were small, and thus power in such subgroup analysis was limited. In addition, the statistically significant results were observed in our multivariable-adjusted analysis only, although there is little statistical evidence for the substantial changes in the effect estimates by any single covariate. Taken together, we cannot exclude the possibility that our statistically significant findings could be caused by chance.
Because WAFACS participants were not screened by colonoscopy at the time of enrollment, some adenomas diagnosed after random assignment may be prevalent at baseline. However, the results did not appreciably change after excluding participants with adenomas that occurred within 2 years after the start of the trial. The WAFACS study design, which included both incident and prevalent adenomas, is potentially more powerful than traditional adenoma recurrence trials to examine the effect on progression of adenomas. Traditional adenoma recurrence trials, which usually have 3–4 years of intervention, are weighted toward small adenomas because recurrent adenomas occur early in the tumorigenic sequence and tend to be small, and the regression rate of small adenomas is high and only a relatively small proportion of adenomas progress. Having prevalent adenomas at enrollment in WAFACS might even be advantageous in studying progression because we had as long as 7.3 years for the intervention to affect progression of adenomas. In WAFACS, consistent with the null results on colorectal cancer reported earlier (14), we found no statistically significant effect of combined B-vitamin treatment on the risk of small, large, or advanced adenomas and the number of adenomas.
It seems unlikely that the doses of folic acid, vitamin B6, and vitamin B12 in our study were insufficient to test the study hypothesis. However, the improved folate status in the general population from the mandatory folic acid fortification (7,8) might have limited the ability of our study to detect an effect. In the WAFACS population (11), the prefortification prevalences of women having inadequate levels of folate (<7ng/mL) were 34.7% and 32.7% in the placebo and treatment group, respectively. After the termination of the trial, only 1.33% had folate levels below 7ng/mL in the placebo group and no one in the active treatment group had levels this low. Mandatory folic acid fortification was associated with an almost 30% elevation in plasma folate levels over a 7-year period (in the placebo group), but the relative increase in folate levels was greater in the active treatment group (69%), in which 49.3% of participants had folate levels greater than 40ng/mL compared with 4.7% in the placebo group. Furthermore, a modest but statistically significantly greater decrease of homocysteine levels was observed in the active treatment group vs the placebo group (18.5% reduction). The statistically significant elevation in plasma folate levels and statistically significant decrease in homocysteine levels by combined folic acid, vitamin B6, and vitamin B12 supplementation were not associated with the overall risk of total colorectal adenoma and advanced colorectal lesions in our study, although we cannot exclude the possibility of a modest effect that might have existed with longer follow-up or if only those with true B-vitamin deficiency were enrolled and treated.
Alcohol is a known folate “antagonist” that affects dietary methyl supply. Alcohol intake has been observed to modify the effect of dietary folate or folic acid supplementation on adenoma risk (1,2). However, we did not observe a statistically significant decrease in the risk of adenoma associated with combined folic acid, vitamin B6, and vitamin B12 treatment in those with higher alcohol intake. Because of relatively low alcohol intake in this population, we were only able to look at the interaction with alcohol at 5g/d or more and thus cannot exclude the possibility that there might be an interaction with alcohol at higher levels. In addition, genetic variations in enzymes involved in one-carbon metabolism may modulate the effect of combined B-vitamin treatment on colorectal neoplasia (25–27). Interindividual genetic variability in our study population is an unlikely explanation for our overall null findings, however, because genetic factors should have been comparable in the active treatment and placebo groups because the participants were randomly assigned to intervention.
The strengths of our study include combined folic acid, vitamin B6, and vitamin B12 supplementation; randomized intervention assignment; sufficient treatment doses; and longer duration of treatment and follow-up. Furthermore, baseline plasma levels of folate, vitamin B6, vitamin B12, homocysteine, cysteine, and cysteinylglycine in this study were assayed so that effect modification by baseline B-vitamin levels could be assessed.
Some limitations of our study also deserve consideration. First, the use of a combination pill did not allow us to investigate the effects of individual components or potential interactions among them in relation to colorectal adenoma. Second, the study population may not be representative of the participants in the parent WAFACS study. However, we found that women included in this analysis had similar baseline characteristics compared with the WAFACS participants and when the randomly assigned groups were compared. Also, the frequencies of endoscopy indications (screening or symptoms) were similarly distributed between the two randomly assigned groups in our study population, and there were no statistically significant effects of combined B-vitamin supplementation on colorectal adenoma risk in the analyses stratified by indications for endoscopies. Third, confounding by extraneous risk factors cannot be completely excluded in our post hoc analysis of randomized trial data, although the baseline characteristics seemed well balanced between the treatment and placebo groups, as is expected in a large-scale trial with effective randomization. Finally, our results were based on women at high risk for CVD and may not be applicable to men or to the general population.
In conclusion, data from this study within a large, randomized, placebo-controlled trial indicate no apparent benefit or harm of combined folic acid, vitamin B6, and vitamin B12 supplementation on the risk of colorectal adenoma among women. Our findings do not support recommending B-vitamin supplementation for the prevention of colorectal adenomas. Further evidence, ideally from large, well-designed randomized controlled trials with long-term follow-up or from large longitudinal studies, is needed to confirm our findings.
Funding
American Cancer Society (RSG-06-263-01-CCE to SMZ); the National Institutes of Health, Bethesda, MD (HL046959 to JEM, CA123089 to SMZ, CA104871 to SMZ, and CA124857 to WC Willett and SMZ).
Notes
The authors would like to thank Martin Van Denburgh (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) for his statistical analytic support and Dr Kana Wu (Harvard School of Public Health, Boston, MA) for her statistical assistance. We acknowledge the contributions of the WAFACS Data Safety and Monitoring Board. We also acknowledge the invaluable contributions of AraSarkissian, Shari Bassuk, and Elaine Zaharris and other WAFACS staff, as well as the 5442 dedicated WAFACS participants.
Vitamin E and its placebo were supplied by Cognis Corporation (LaGrange, IL). All other agents and their placebos were supplied by BASF Corporation (Mount Olive, NJ). Pill packaging was provided by Cognis and BASF. The American Cancer Society, National Institutes of Health, Cognis, and BASF did not provide any input into the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
References
- 1. Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review J Nutr 2002. 132(8Suppl):2350S–2355S [DOI] [PubMed] [Google Scholar]
- 2. Kim YI. Folate and colorectal cancer: an evidence-based critical review Mol Nutr Food Res 2007. 51(3):267–292 [DOI] [PubMed] [Google Scholar]
- 3. Lee JE, Willett WC, Fuchs CS, et al. Folate intake and risk of colorectal cancer and adenoma: modification by time Am J Clin Nutr 2011. 93(4):817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies JAMA 2010. 303(11):1077–1083 [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim EM, Zekri JM. Folic acid supplementation for the prevention of recurrence of colorectal adenomas: metaanalysis of interventional trials Med Oncol 2010. 27(3):915–918 [DOI] [PubMed] [Google Scholar]
- 6. Figueiredo JC, Mott LA, Giovannucci E, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials Int J Cancer 2011. 129(1):192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Center for Disease Control and Prevention Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects MMWR Morb Mortal Wkly Rep 1992. 41(No. RR-14):1–7 [Google Scholar]
- 8. Food and Drug Administration, Dept of Health and Human Services. Food standards amendament of standards of identity for enriched grain products to require addition of folic acid. Final rule. 21 CFR Parts 136, 137, and 139 Fedral Register 1996. 61 8781–8797 [Google Scholar]
- 9. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006 J Nutr 2011. 141(2):261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006 Am J Clin Nutr 2010. 91(1):231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial JAMA 2008. 299(17):2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassuk SS, Albert CM, Cook NR, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants J Womens Health (Larchmt) 2004. 13(1):99–117 [DOI] [PubMed] [Google Scholar]
- 13. Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study Arch Intern Med 2007. 167(15):1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang SM, Cook NR, Albert CM, et al. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial JAMA 2008. 300(17):2012–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu K, Platz EA, Willett WC, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma Am J Clin Nutr 2009. 90(6):1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin-Buehring YS, Stuempfig L, Pouget E, et al. Characterization of galactose-1-phosphate uridyl-transferase and galactokinase in human organs from the fetus and adult Clin Chim Acta 1981. 112(3):257–265 [DOI] [PubMed] [Google Scholar]
- 17. Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection J Chromatogr 1987. 422 43–52 [DOI] [PubMed] [Google Scholar]
- 18. Barthel FM, Babiker A, Royston P, et al. Evaluation of sample size and power for multi-arm survival trials allowing for non-uniform accrual, non-proportional hazards, loss to follow-up and cross-over Stat Med 2006. 25(15):2521–2542 [DOI] [PubMed] [Google Scholar]
- 19. Matsubara K, Komatsu S, Oka T, et al. Vitamin B6-mediated suppression of colon tumorigenesis, cell proliferation, and angiogenesis (review) J Nutr Biochem 2003. 14(5):246–250 [DOI] [PubMed] [Google Scholar]
- 20. Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial JAMA 2007. 297(21):2351–2359 [DOI] [PubMed] [Google Scholar]
- 21. Logan RF, Grainge MJ, Shepherd VC, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas Gastroenterology 2008. 134(1):29–38 [DOI] [PubMed] [Google Scholar]
- 22. Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr 2008. 87(3):517–533 [DOI] [PubMed] [Google Scholar]
- 23. Ulrich CM. Folate and cancer prevention: a closer look at a complex picture Am J Clin Nutr 2007. 86(2):271–273 [DOI] [PubMed] [Google Scholar]
- 24. Ulrich CM, Potter JD. Folate and cancer—timing is everything. JAMA 2007. 297(21):2408–2409 [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Giovannucci E, Hankinson SE, et al. A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma Carcinogenesis 1998. 19(12):2129–2132 [DOI] [PubMed] [Google Scholar]
- 26. Levine AJ, Siegmund KD, Ervin CM, et al. The methylenetetrahydrofolate reductase 677C-->T polymorphism and distal colorectal adenoma risk Cancer Epidemiol Biomarkers Prev 2000. 9(7):657–663 [PubMed] [Google Scholar]
- 27. Ulrich CM, Kampman E, Bigler J, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev 1999. 8(8):659–668 [PubMed] [Google Scholar]


