Abstract
Background
The contribution of multiple retained nonfunctional arteriovenous grafts (AVGs) to the burden of chronic inflammation in chronic hemodialysis patients has not been well studied. Here, we sought to evaluate the association between plasma levels of C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-alpha) and albumin and the number of retained nonfunctional AVGs.
Methods
This cross-sectional study enrolled 91 prevalent patients undergoing in-center hemodialysis without evidence of infection or inflammation. A baseline blood sample was obtained at study enrollment. A general linear model (GLM) was used to compare levels of biomarkers of systemic inflammation across groups defined by the number of retained, nonfunctional AVGs.
Results
A total of 43 patients had one or more retained thrombosed AVG and had significantly greater plasma log-CRP levels compared with patients without a previous AVG (P= 0.036), regardless of the current AV access type. Using a GLM, we found that for every additional retained thrombosed AVG, plasma log-CRP, log-IL-6 and TNF-alpha concentrations increased significantly by 0.30 mg/L (P= 0.011), 0.18 pg/mL (P= 0.046) and 0.72 pg/mL (P= 0.046), respectively, following adjustment.
Conclusions
Hence, the severity of inflammation increases with the number of retained nonfunctional AVG's, suggesting that AVG accumulation may contribute to the cardiovascular morbidity and mortality associated with chronic inflammation in asymptomatic end-stage renal disease (ESRD) patients. Further study is indicated to determine whether patients with one or more thrombosed, retained AVG may benefit from periodic screening with CRP monitoring to identify those patients who may benefit from AVG resection.
Keywords: AVG, ESRD, hemodialysis, inflammation
INTRODUCTION
In 2007, among prevalent end-stage renal disease (ESRD) hemodialysis patients, the rate of infection of a functioning dialysis arteriovenous graft (AVG) was 0.39 events per patient year, more than two times higher than that among arteriovenous fistula (AVF) patients [1], resulting in bacteremia, septicemia and metastatic bacterial infection. Although no longer functional, thrombosed, retained AVGs are also a potential nidus for infection and a source of systemic inflammation, as originally described by Ayus et al. [2, 3] It has been estimated that >35% of prevalent ESRD patients have a minimum of one retained thrombosed AVG [3].
Currently, unless overt clinical signs or symptoms indicate a localized AVG infection, thrombosed AVGs are retained within the ESRD patient's extremity, often resulting in multiple retained nonfunctional AVGs. Occult infection of a thrombosed AVG may be identified only once the patient develops bacteremia. In this setting, infected AVGs have been detected by indium-labeled white blood cell scan, [4–6] and in reported cases, AVG resection and antibiotic therapy have led to resolution of bacteremia [3, 6]. Limited studies suggest that occult AVG infection is associated with erythropoietin (EPO) resistance, hypoalbuminemia and elevated C-reactive protein (CRP), [7, 8], all of which are reported to significantly improve with AVG resection [3]. It has not yet been examined whether the accumulation of retained thrombosed AVGs correlates with the severity of chronic inflammation among asymptomatic ESRD patients, and thereby contributes to adverse patient outcomes.
We examined the relationship between markers of chronic inflammation and the number of retained thrombosed AVGs among a cohort of prevalent ESRD patients receiving in-center hemodialysis.
MATERIALS AND METHODS
Study population
Adult ESRD patients receiving in-center, thrice-weekly, maintenance hemodialysis at one of six Emory University-affiliated Davita dialysis centers, and who had undergone AV access evaluation following referral by their nephrologist at the Emory Dialysis Access Center of Atlanta between September 2006 to November 2008, were eligible for study enrollment, as previously described [9]. This observational study was designed to examine the association of novel thrombotic and inflammatory biomarkers with AV access type and outcomes, and included subjects with and without a history of thrombotic occlusion of a functioning arteriovenous (AV) access. AV access thrombosis was defined as the absence of blood flow to the AV access site and the inability to use it for dialysis. A large proportion of patients within the Emory dialysis centers are Black; of the 94 patients prospectively enrolled, 91 were Black, and thus, our analysis was limited to black patients. Patients were excluded from the study for conditions that reflect a state of inflammation or for use of anti-inflammatory therapy, including (i) presence of known malignancy or active vasculitis; (ii) evidence of local or systemic infection or inflammation or (iii) current or recent use of steroids, calcineurin inhibitors or antimetabolite medications (methotrexate, azathioprine, 5-flurouracil, mercaptopurine, sulfadiazine). Patients were also excluded if they had clinical signs or symptoms of graft-site or other infection, or were using a central venous catheter for dialysis.
At study enrollment, direct patient interview and medical record review were conducted to collect clinical and dialysis treatment data, and a baseline blood sample was obtained. During physical examination, vascular access type currently used and the number of previous AVGs and AVFs were determined and confirmed by reviewing medical and surgical records for AV access insertions, revisions and resections. The Institutional Review Board (IRB) of Emory University Medical Center approved the study protocol. Informed consent was obtained from each patient prior to study enrollment. Human subjects' protocol followed were in accordance with the ethical standards of the institutional IRB and with the Helsinki Declaration of 1975.
Definition of variables
All assessments were obtained at a single baseline visit. Covariates including age, gender, self-reported race, and length of time on dialysis, body mass index (BMI), smoking status (never, former, current) and vascular access type (AVF, AVG, central venous catheter) were collected. Comorbidies included diabetes (defined by use of diabetes medications), hypertension, congestive heart failure, ischemic heart disease (which included myocardial infarction, angina or coronary intervention), stroke or transient ischemic event and peripheral arterial disease (PAD). Results of albumin and hemoglobin obtained within two weeks of the baseline blood sample measurement were abstracted from the outpatient dialysis records and included in the analysis. EPO resistance was defined as the weekly EPO dose/hematocrit ratio [10].
Serum cytokine measurement
All the patients received outpatient hemodialysis therapy three times per week. A blood sample was obtained from each patient before a routine, midweek hemodialysis session within 5 days of study enrollment. Following collection, serum and plasma were aliquoted and stored at −80°C for subsequent analysis. All assays were performed as directed by the manufacturer and samples were centrifuged for 10 min at 10 000 rpm before testing. All the assays had quality controls, plasma controls and a non-plasma sample that was added to each plate. The dilution of the samples (always done with the Calibration diluent in which the Standard Cocktail was reconstituted) depended on the individual assays and of the expected concentration range for the samples tested. The Fluorokine® MultiAnalyte Profiling (MAP) Kits from R&D Systems (Minneapolis, USA) were used to determine the levels of analytes. Interleukin (IL)-6 level was measured with the Fluorokine® MAP MultiAnalyte Profiling Human Base Kit A (R&D Systems) (the inter-assay variability is 4.6%). The same Base Kit A was used to determine the concentration of tumor necrosis factor alpha (TNF-alpha) (the inter-assay variability is 3.0%). high-sensitivity C-reactive protein levels were quantified using the Dade-Behring Nephelometry System-BNII.
Statistical analysis
Patients' characteristics were summarized and compared between groups stratified by the number of previous AVGs. Patient age, length of time on dialysis, BMI and hemoglobin were presented with mean (standard error) and compared using Welch ANOVA, which allows unequal variances between groups. Categorical variables, such as sex, smoking status, type of AV access and diabetes, were compared using Fisher exact tests.
A general linear model (GLM) was employed to compare biomarkers of systemic inflammation (CRP, IL-6, TNF-alpha, and serum albumin) that were treated as continuous variables and used to test the significance of differences in these biomarker values across different groups defined by the number of retained nonfunctional AVGs and AV access sites. The means of each biomarker of systemic inflammation within groups were stratified by the number of previous AVGs or AV access sites and were also depicted in figures. The CRP and IL-6 data were skewed and not normally distributed, and therefore, they were log-transformed before normality-based analyses, including ANOVA and linear regression, were applied.
GLM was used to estimate the unadjusted, partially adjusted and fully adjusted associations between each marker of systemic inflammation and retained/prior AV access. Partial adjustment controlled only for the length of time on dialysis, age and type of AV access. Full adjustment controlled for age, sex, BMI, smoking status, diabetes, length of time on dialysis and type of AV access. GLM was also used to estimate the association between each biomarker of systemic inflammation and current AVG/AVF use, while controlling for prior AVG use and AVF use. The significance levels were set at 0.05 for all the tests. The SAS statistical package (SAS Institute, Inc., Cary, NC) was used for all data managements and analyses.
RESULTS
The study participants (n = 91) had an average age of 59 years, with 47% of the cohort male, 100% Blacks and an average length of time on dialysis of 5.7 years (Table 1). Among the participants, the average patient BMI was 29.1 ± 6.9 kg/m2, 49% had diabetes, 98% had hypertension, 44% had a history of cardiovascular disease, 22% peripheral vascular disease, 4% had a hypercoagulable state, 39% had a history of smoking, and 49% of participants used an AVG and 51% used an AVF for hemodialysis at study enrollment.
Table 1.
Baseline characteristics of study participants by number of retained thrombosed AVGs
| All patients (n = 91) | Number of previous AVGs |
P-value | |||
|---|---|---|---|---|---|
| 0 (n = 48) | 1–2 (n = 33) | 3+ (n = 10) | |||
| Age (years) | 59.3 ± 12.4 | 62.3 ± 12.5 | 56.8 ± 10.6 | 52.6 ± 14.2 | 0.054 |
| Sex | |||||
| Female | 48 (53%) | 23 (48%) | 18 (55%) | 7 (70%) | 0.43 |
| Male | 43 (47%) | 25 (52%) | 15 (45%) | 3 (30%) | |
| Dialysis vintage (years) | 5.7 ± 4.2 | 3.9 ± 2.8 | 7.2 ± 4.5 | 9.6 ± 4.1 | <0.001 |
| Body mass index (kg/m2) | 29.1 ± 6.9 | 29.0 ± 6.6 | 29.2 ± 7.2 | 29.6 ± 8.2 | 0.98 |
| Primary renal disease | |||||
| Diabetes | 37 (41%) | 22 (46%) | 13 (39%) | 2 (20%) | 0.22 |
| Hypertension | 40 (44%) | 21 (44%) | 12 (36%) | 7 (70%) | |
| Othera | 14 (15%) | 5 (10%) | 8 (24%) | 1 (10%) | |
| Comorbidities | |||||
| Diabetes | 45 (49%) | 25 (52%) | 14 (42%) | 6 (60%) | 0.57 |
| Hypertension | 89 (98%) | 47 (98%) | 32 (97%) | 10 (100%) | 1.00 |
| History of CVDb | 40 (44%) | 23 (48%) | 13 (39%) | 4 (40%) | 0.75 |
| Peripheral Diseasec | 20 (22%) | 13 (27%) | 5 (15%) | 2 (20%) | 0.47 |
| Hypercoagulability | 4 (4%) | 3 (6%) | 1 (3%) | 0 (0%) | 0.78 |
| Tobacco use | |||||
| Never | 55 (60%) | 30 (63%) | 18 (55%) | 7 (70%) | 0.91 |
| Former | 23 (25%) | 12 (25%) | 9 (27%) | 2 (20%) | |
| Current | 13 (14%) | 6 (12%) | 6 (18%) | 1 (10%) | |
| Current vascular access | |||||
| AVG | 45 (49%) | 19 (40%) | 19 (58%) | 7 (70%) | 0.10 |
| AVF | 46 (51%) | 29 (60%) | 14 (42%) | 3 (30%) | |
| Erythropoietin use | 74 (81%) | 40 (83%) | 26 (79%) | 8 (80%) | 0.93 |
| Hemoglobin (g/dL) | 12.1 ± 1.4 | 12.2 ± 1.4 | 12.2 ± 1.5 | 11.7 ± 1.2 | 0.53 |
Results are expressed as mean ± SD
aOther includes hereditary, ischemic or glomerulonephritis and unknown causes.
bIncludes ischemic heart disease, myocardial infarction, CABG, CHF, angina, arrhythmia.
cIncludes peripheral vascular disease, history of lower extremity angioplasty or bypass surgery, history of claudication. Significant P-values are indicated in bold.
Among the overall cohort, 67% (61) patients had a previous permanent AV access (either an AVF or AVG) and 47% of patients had one or more retained AVG; of these, 77% had 1–2 thrombosed, retained AVG and 23% had 3 or more thrombosed, retained AVG. Of the patients currently using an AVG, 58% had a history of one or more retained AVG, while among patients using an AVF, 37% had one or more retained AVG. Patient characteristics associated with one or more thrombosed, retained AVG included length of time on dialysis, which was significantly longer among patients with one or more AVG (P< 0.001) compared with patients with none. Of marginal significance was patient age (P= 0.054), as younger patients tended to have had one or more retained thrombosed AVG. There were no significant differences in gender, BMI, primary renal disease, comorbidities, tobacco use, current type of AV access, EPO use or serum hemoglobin among patients with 0, 1–2 or 3+ previous, thrombosed, retained AVGs (Table 1).
Upon stratification of inflammatory biomarkers by the number of thrombosed, retained AVGs, in general, their concentrations were greater as the number of retained AVGs increased from 0 to 3+ (Figure 1), although these differences did not reach statistical significance. In contrast, patients with a history of one or more nonfunctional, retained AVG had significantly greater log-CRP concentrations compared with patients who had never had an AVG (1.68 mg/L versus 1.17 mg/L, P= 0.045), while no significant difference was observed between groups in log-IL-6, TNF-alpha, or serum albumin concentrations (data not shown).
FIGURE 1:
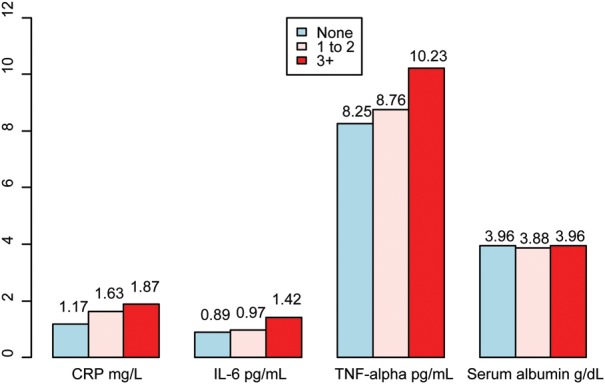
Mean inflammatory biomarker concentration stratified by the number of retained thrombosed AVGs (none, 1–2, 3+), where CRP and IL-6 are log-transformed.
Figure 2 shows the unadjusted, partially adjusted and fully adjusted effects of thrombosed, retained AVGs on plasma inflammatory biomarker concentrations, where biomarkers with a skewed distribution were log-transformed. In the unadjusted model, each additional retained AVG was significantly associated with an increase in the plasma concentrations of log-CRP and TNF-alpha of 0.25 mg/L (P= 0.014) and 0.57 pg/mL (P= 0.048), respectively, while there was no significant increase in log-IL-6 concentration (P= 0.13). After adjusting for age, length of time on dialysis and current type of AV access in the partially adjusted model, for every additional retained AVG, the log-CRP concentration significantly increased by 0.35 mg/L (P= 0.003), while no significant change occurred in log-IL-6 concentration (P= 0.058) or in TNF-alpha concentration (P= 0.11). Finally, in the fully adjusted model, controlling for sex, BMI, smoking status and diabetes, each additional retained thrombosed AVG accounted for a significant increase in plasma log-CRP (0.30 mg/L, P= 0.011), log-IL-6 (0.18 pg/mL, P= 0.046) and TNF-alpha (0.72 pg/mL, P= 0.046). There was no significant change in serum albumin concentration as the number of retained AVGs increased in the adjusted models.
FIGURE 2:
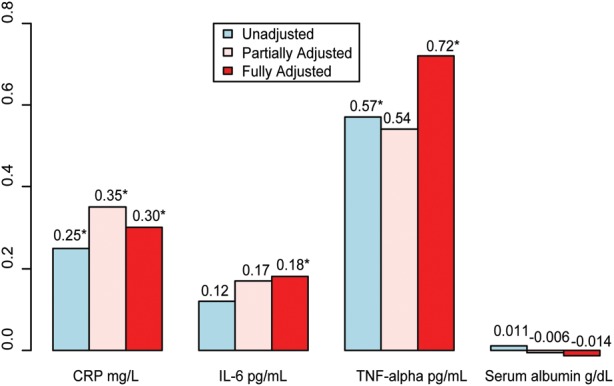
Change in inflammatory biomarker level for each additional retained thrombosed AVG, treated as a continuous variable, where CRP and IL-6 are log-transformed. Partially Adjusted: adjusted for age, length of time on dialysis and type of AV access (AVF versus Other). Fully Adjusted: adjusted for age, sex, body mass index, smoking status, diabetes, length of time on dialysis and type of AV access (AVF versus Other). *P-value < 0.05.
To further examine the relationship between the number and type of previous vascular access to inflammation, patients were stratified by current AVF versus AVG use, prior AVG use and prior AVF use (Table 2), and regression coefficient estimates representing group differences were calculated. The plasma concentrations of log-CRP, log-IL-6, and TNF-alpha were not statistically different between patients currently using an AVG versus AVF, or those whose vascular access history was limited to AVF use. In contrast, current AVG use had a significant negative effect on serum albumin concentration compared with current AVF use, and was 0.20 g/dL lower among current AVG users (P= 0.032). When comparing the effect of one or more retained thrombosed AVG versus none on log-CRP, log-IL-6, TNF-alpha and serum albumin, log-CRP concentration was significantly greater (0.58 mg/L) among patients with a retained nonfunctional AVG (P= 0.036). There was no significant difference in inflammatory biomarkers between patients with prior AVF use versus none.
Table 2.
Estimated difference in inflammatory biomarker between groups defined by current AVG/AVF use, prior AVG use (0 versus 1+) and prior AVF use (0 versus 1+)
| Group | Inflammatory biomarker |
|||
|---|---|---|---|---|
| Log CRP mg/L | Log IL-6 pg/mL | TNF-alpha pg/mL | Serum albumin g/dL | |
| Current AVG versus AVF use | −0.34 (0.20) | −0.18 (0.40) | 0.88 (0.27) | −0.20 (0.032) |
| Prior, retained AVG (0 versus 1+) | 0.58 (0.036) | 0.23 (0.28) | 0.55 (0.50) | −0.009 (0.92) |
| Prior AVF use (0 versus 1+) | 0.27 (0.32) | 0.19 (0.37) | −0.035 (0.97) | 0.062 (0.49) |
P-values are shown in parentheses. Significant P-values are indicated in bold.
Finally, we examined the relationship between inflammation and EPO resistance, defined as the weekly EPO dose/ hematocrit ratio [10]. Following adjustment for age, sex, BMI, smoking status, diabetes, length of time on dialysis and type of AV access (AVF versus others), there was a statistically significant association between EPO resistance and greater plasma log-CRP (P= 0.003) and log IL-6 concentrations (P= 0.003).
DISCUSSION
In this study we showed that plasma CRP, IL-6 and TNF-alpha concentrations significantly correlate with the number of retained thrombosed AVGs in ESRD patients who lack clinical evidence of infection or inflammation. Furthermore, we showed that elevated CRP and IL-6 concentrations observed in conjunction with the accumulation of nonfunctional AVGs are significantly associated with EPO resistance. These findings provide further evidence that the accumulation of retained nonfunctional AVGs may contribute to cardiovascular morbidity and cardiovascular and all-cause mortality associated with chronic inflammation in CKD and ESRD patients [11–14].
Previously, Nassar et al. [3] reported that occult infection of a single nonfunctioning AVG is associated with elevated CRP. In their study, 212 patients were screened for chronic inflammation, indicated by the presence of EPO resistance, elevated CRP and serum albumin concentrations < 3.3 g/dL; eight patients were found to have a chronic inflammatory state and a single retained nonfunctioning AVG. Of these, six patients showed evidence of infection around the nonfunctioning AVG by indium-labeled white blood cell scan. All six grafts were resected, and demonstrated peri-graft purulent material containing bacteria. Within two months of AVG resection, EPO resistance declined significantly, and plasma CRP was significantly lower than at baseline. Our results extend those of Nassar et al. by finding that CRP, IL-6 and TNF-alpha concentrations significantly increase with the addition of each retained thrombosed AVG after controlling for confounding factors. In addition, we showed that patients with a history of one or more retained thrombosed AVG have a significantly greater CRP level, regardless of the type of AV access currently in use. In accordance with Nassar et al., we found that EPO resistance was present in the setting of higher concentrations of CRP and IL-6 associated with a greater number of retained thrombosed AVGs.
In our study, participants with one or more thrombosed, retained AVG tended to be younger and had received dialysis treatment for a significantly greater length of time than patients with no history of AVG use. These factors may account for the higher prevalence of multiple retained AVGs (47%) in our patient population than has been previously estimated (35%) [3]. We cannot confirm that these factors explained our findings, however, as demographic data from previous reports are limited [2, 3]. We also found that 49% of our prevalent patient cohort were using an AVG at the time of enrollment, comparable to a study of nearly 80 000 prevalent Fresenius hemodialysis patients from the same study period, in which 48.9% of black patients were using an AVG [11]. Black ESRD patients are more likely to receive an AVG and less likely to use an AVF [15, 16], which may contribute to the prevalence of AVG use in our study cohort. Interestingly, 37% of patients with current AVF use had a history of one or more nonfunctional, retained AVG, suggesting the opportunity for secondary AVF creation despite previous AVG placement.
The presence of chronic inflammation is multifactorial [17] and well described in ESRD patients [18], yet the contribution of non-autologous retained material to underlying chronic inflammation is less well known. The accumulation of retained thrombosed AVGs may independently contribute to inflammation by serving as nidi for bacteria that enter the bloodstream during cannulation of a functioning AV access, via a central venous catheter [19], or through skin breakdown. Another potential source of inflammation in ESRD is the presence of a failed renal allograft [20], which if removed, confers a significant survival benefit [21]. We believe that few patients in our study had a history of a failed renal allograft, and this factor was not part of our exclusion criteria.
As a means of risk ascertainment, the National Kidney Foundation K/DOQI guidelines recommend periodic assessment of CRP levels in dialysis patients, and although a firm cut-point has not been established, [22] suggest that CRP levels in excess of 5–10 mg/L define the presence of inflammation in CKD. However, national guidelines do not make recommendations on the frequency of CRP monitoring and there is no consensus regarding treatment of chronic inflammation in CKD patients. Therefore, the practitioner is left to his/her best clinical judgment.
Given the contribution of thrombosed, nonfunctional AVGs to chronic inflammation, we believe that a reasonable approach is to obtain CRP levels on two separate occasions, 2–4 weeks apart, in patients with a history of recurrent bacteremia, hypoalbuminemia or EPO resistance who also have one or more retained thrombosed AVG. If the CRP level is elevated at each interval, investigation of possible sources of underlying inflammation is warranted. Indium-111 leukocyte imaging is the most sensitive and specific (85–90%) radionuclide imaging tool for detecting infection of a peripheral arm AVG, and requires only an IV catheter for withdrawal of blood and reinjection of labeled cells [23]. Because persistently elevated CRP levels are associated with higher morbidity and overall mortality, resection of the AVG is indicated if the indium-white blood cell scan shows positive uptake, which can result in a reduction in CRP levels and improved EPO responsiveness [3].
The limitations of this study bear mention. Given the observational nature of this study, there is risk of residual confounding. To minimize this, we adjusted estimates for multiple factors that may confound the relationship between AVG number and inflammation such as demographics, AV access type in use and comorbid conditions. In addition, we excluded patients using a central venous catheter and those with evidence of local or systemic infection or with conditions known to reflect a state of inflammation. Of note, we recruited patients from a single dialysis chain, and in doing so, attempted to minimize variations in policies and procedures related to dialyzer use and dialyzate, which are potential sources of cytokine production. Second, our study is cross-sectional and CRP, IL-6 and TNF- alpha were assessed using a single measurement, resulting in the potential for misclassification of patients with acute inflammation as having chronic inflammation. However, previous studies suggest a sustained elevation of CRP levels in CKD patients [24] and support use of a single CRP measurement for short-term (up to one year) risk assessment [17]. Finally, because of the relatively small sample size, it is not possible to extend our findings to examine patient mortality. Nevertheless, multiple adverse outcomes are associated with chronic inflammation as reflected by elevated CRP and IL-6, including carotid artery atherosclerosis [25], myocardial infarction [14], cardiovascular and all-cause mortality [2, 11–13, 26, 27].
In conclusion, our data demonstrate a significant and graded association between the number of retained nonfunctional AVGs and biomarkers of chronic inflammation, raising the possibility that the accumulation and retention of thrombosed AVGs contributes to adverse patient outcomes. ESRD patients with one or more thrombosed, retained AVG should be considered as being at increased risk of chronic inflammation and may benefit from periodic screening with CRP monitoring to better identify those patients who may benefit from resection of nonfunctional AVGs.
CONFLICT OF INTEREST STATEMENT
None declared. (See related article by Achinger and Ayus. Inflammation from dialysis, can it be removed? Nephrol Dial Transplant 2013; 28: 770–773.)
ACKNOWLEDGMENTS
This study was supported by an NIH K23 Grant DK65634 (H.W.), a Davita Clinical Research Grant (H.W.) and a PHS Grant (UL1 RR02008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
REFERENCES
- 1.U.S. Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Ayus JC, Sheikh-Hamad D. Silent infection in clotted hemodialysis access grafts. J Am Soc Nephrol. 1998;9:1314–1317. doi: 10.1681/ASN.V971314. [DOI] [PubMed] [Google Scholar]
- 3.Nassar GM, Fishbane S, Ayus JC. Occult infection of old nonfunctioning arteriovenous grafts: a novel cause of erythropoietin resistance and chronic inflammation in hemodialysis patients. Kidney Int Suppl. 2002;80:49–54. doi: 10.1046/j.1523-1755.61.s80.10.x. [DOI] [PubMed] [Google Scholar]
- 4.Palestro CJ, Vega A, Kim CK, et al. Indium-111-labeled leukocyte scintigraphy in hemodialysis access-site infection. J Nucl Med. 1990;31:319–324. [PubMed] [Google Scholar]
- 5.Lawrence PF, Dries DJ, Alazraki N, et al. Indium 111-labeled leukocyte scanning for detection of prosthetic vascular graft infection. J Vasc Surg. 1985;2:165–173. [PubMed] [Google Scholar]
- 6.Nassar GM, Ayus JC. Infectious complications of old nonfunctioning arteriovenous grafts in renal transplant recipients: a case series. Am J Kidney Dis. 2002;40:832–836. doi: 10.1053/ajkd.2002.35696. [DOI] [PubMed] [Google Scholar]
- 7.Canaud B, Senecal L, Leray-Moragues H, et al. Vascular access, an underestimated source of inflammation in dialysis patients. Nephrologie. 2003;24:353–358. [PubMed] [Google Scholar]
- 8.Kaysen GA. Inflammation: cause of vascular disease and malnutrition in dialysis patients. Semin Nephrol. 2004;24:431–436. doi: 10.1016/j.semnephrol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Wasse H, Cardarelli F, De Staercke C, et al. 25-hydroxyvitamin D concentration is inversely associated with serum MMP-9 in a cross-sectional study of African American ESRD patients. BMC Nephrol. 2011;12:24. doi: 10.1186/1471-2369-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locatelli F, Andrulli S, Memoli B, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21:991–998. doi: 10.1093/ndt/gfk011. [DOI] [PubMed] [Google Scholar]
- 11.Lacson E, Jr., Wang W, Lazarus JM, et al. Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2009;54:912–921. doi: 10.1053/j.ajkd.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Achinger SG, Mizani MR, Ayus JC. Use of 3-hour daily hemodialysis and paricalcitol in patients with severe secondary hyperparathyroidism: A case series. Hemodial Int. 2010;14:193–199. doi: 10.1111/j.1542-4758.2009.00424.x. [DOI] [PubMed] [Google Scholar]
- 14.Moritz ML, Ayus JC. Improving intravenous fluid therapy in children with gastroenteritis. Pediatr Nephrol. 2010;25:1383–1384. doi: 10.1007/s00467-010-1505-2. [DOI] [PubMed] [Google Scholar]
- 15.Wasse H, Hopson SD, McClellan W. Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol. 2010;32:234–241. doi: 10.1159/000318152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasse H, Speckman RA, Frankenfield DL, et al. Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis. 2007;49:276–283. doi: 10.1053/j.ajkd.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuwese CL, Stenvinkel P, Dekker FW, et al. Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat Rev Nephrol. 2011;7:166–176. doi: 10.1038/nrneph.2011.2. [DOI] [PubMed] [Google Scholar]
- 18.Kaysen GA, Eiserich JP. Characteristics and effects of inflammation in end-stage renal disease. Semin Dial. 2003;16:438–446. doi: 10.1046/j.1525-139x.2003.16096.x. [DOI] [PubMed] [Google Scholar]
- 19.Wasse H. Catheter-related mortality among ESRD patients. Semin Dial. 2008;21:547–549. doi: 10.1111/j.1525-139X.2008.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayus JC, Achinger SG. At the peril of dialysis patients: ignoring the failed transplant. Semin Dial. 2005;18:180–184. doi: 10.1111/j.1525-139X.2005.18304.x. [DOI] [PubMed] [Google Scholar]
- 21.Ayus JC, Achinger SG, Lee S, et al. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21:374–380. doi: 10.1681/ASN.2009050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:A1–A6. [PubMed] [Google Scholar]
- 23.Taylor Andrew SD, Naomi Alazraki. A Clinican's Guide to Nuclear Medicine. Second edn. Reston, VA: The Society of Nuclear Medicine: 2006. [Google Scholar]
- 24.Deneuville M. Infection of PTFE grafts used to create arteriovenous fistulas for hemodialysis access. Ann Vasc Surg. 2000;14:473–479. doi: 10.1007/s100169910090. [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P, Heimburger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis. 2002;39:274–282. doi: 10.1053/ajkd.2002.30546. [DOI] [PubMed] [Google Scholar]
- 26.Moritz ML, Ayus JC. 100 cc 3% sodium chloride bolus: a novel treatment for hyponatremic encephalopathy. Metab Brain Dis. 2010;25:91–96. doi: 10.1007/s11011-010-9173-2. [DOI] [PubMed] [Google Scholar]
- 27.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]


