Abstract
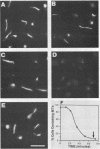
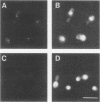
Overexpression of alpha- and beta-tubulin genes in Saccharomyces cerevisiae, separately or together, leads to accumulation of large excesses of each of the polypeptides and arrest of cell division. However, other consequences of overexpression of these genes differ in several ways. As shown previously (D. Burke, P. Gasdaska, and L. Hartwell, Mol. Cell. Biol. 9:1049-1059, 1989), overexpression of beta-tubulin leads, at early times, to loss of microtubule structures and loss of viability. Eventually, the excess beta-tubulin forms abnormal structures. We show here that, in contrast, overexpression of alpha-tubulin led to none of these phenotypes and in fact could suppress each of the phenotypes associated with beta-tubulin accumulation. Truncated forms of beta-tubulin that were not competent to carry out microtubule functions also failed to elicit the beta-tubulin-specific phenotypes when overexpressed. The data support the hypothesis that beta-tubulin in excess over alpha-tubulin is uniquely toxic, perhaps because it interferes with normal microtubule assembly.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Borisy G. G. Structural polarity and directional growth of microtubules of Chlamydomonas flagella. J Mol Biol. 1974 Dec 5;90(2):381–402. doi: 10.1016/0022-2836(74)90381-7. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Fridovich-Keil J. L., Pillus L., Mulligan R. C., Solomon F. A chicken-yeast chimeric beta-tubulin protein is incorporated into mouse microtubules in vivo. Cell. 1986 Feb 14;44(3):461–468. doi: 10.1016/0092-8674(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Atkins J. F., McGill C., Chow L. Identification and mapping of the transcriptional and translational products of the yeast plasmid, 2mu circle. Cell. 1979 Apr;16(4):827–839. doi: 10.1016/0092-8674(79)90098-9. [DOI] [PubMed] [Google Scholar]
- Burke D., Gasdaska P., Hartwell L. Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1049–1059. doi: 10.1128/mcb.9.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988 Sep;13(9):339–343. doi: 10.1016/0968-0004(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Guarente L., Yocum R. R., Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. W., Adams A. E., Szaniszlo P. J., Pringle J. R. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1988 Oct;107(4):1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz W. S., Solomon F. Diversity among beta-tubulins: a carboxy-terminal domain of yeast beta-tubulin is not essential in vivo. Mol Cell Biol. 1988 Jul;8(7):2730–2736. doi: 10.1128/mcb.8.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz W., Weinstein B., Solomon F. Regulation of tubulin levels and microtubule assembly in Saccharomyces cerevisiae: consequences of altered tubulin gene copy number. Mol Cell Biol. 1990 Oct;10(10):5286–5294. doi: 10.1128/mcb.10.10.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Giveon D., Thierauf M., Ginzburg I., Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Kunes S., Schatz P. J., Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58(2-3):201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Paschal B. M., Obar R. A., Vallee R. B. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature. 1989 Nov 30;342(6249):569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- Pillus L., Solomon F. Components of microtubular structures in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2468–2472. doi: 10.1073/pnas.83.8.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Georges G. E., Solomon F., Botstein D. Insertions of up to 17 amino acids into a region of alpha-tubulin do not disrupt function in vivo. Mol Cell Biol. 1987 Oct;7(10):3799–3805. doi: 10.1128/mcb.7.10.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Pillus L., Grisafi P., Solomon F., Botstein D. Two functional alpha-tubulin genes of the yeast Saccharomyces cerevisiae encode divergent proteins. Mol Cell Biol. 1986 Nov;6(11):3711–3721. doi: 10.1128/mcb.6.11.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry. 1984 Sep 25;23(20):4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Neff N. F., Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]