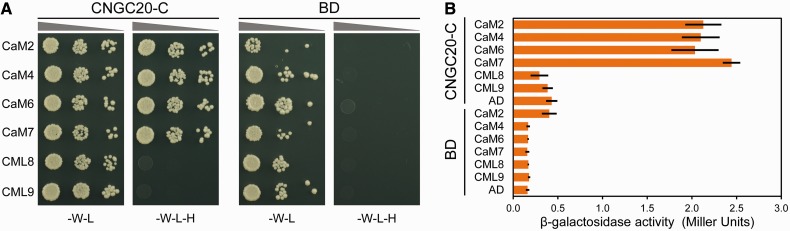
Fig. 1.
The C-terminus of CNGC20 interacts with calmodulins but not calmodulin-like proteins. (A) Left columns: the CNGC20 C-terminus (CNGC20-C) fused to the GAL4-binding domain (BD) was used in the YTH assay together with CaM isoforms or the CaM-like proteins CML8 and CML9, which were fused to the GAL4-activation domain (AD). Right columns: experiments repeated using the BD without CNGC20-C. Yeasts were grown in the absence of tryptophan (–W) and leucine (–L) for selection of co-transformed cells, and in the absence of histidine (–H), tryptophan (–W) and leucine (–L) to monitor protein interactions. (B) Interaction between CNGC20-C and CaM isoforms, CML8 and CML9 was quantified using the β-galactosidase activity assay. Bars represent mean results of two independent measurements with three replicates each. Labels as in (A).