Abstract
Background & objectives:
Bio-manipulation technique is of primary importance during the development of transgenic mosquitoes. The study describes the variable factors that influence the viability of medically important mosquito vectors during microinjection.
Methods:
Three mosquito vectors belonging to the genus Aedes, Anopheles and Culex were microinjected at different developmental stages of their life cycle viz., egg, larvae, pupae and adult.
Results:
The improvisations revealed an increased survivability of biomanipulated mosquitoes during the embryonic and adult microinjection. The study of injecting larvae and pupae resulted in poor survivability.
Interpretation & conclusions:
The microinjection protocol was successfully tested on three important mosquito vectors. The critical period after biomanipulation which contributes heavily for the survivability factor was evaluated. The results provide a common protocol for biomanipulation of three mosquito vectors with enhanced survivability.
Keywords: Bio-manipulation, critical period, microinjection, mosquito vectors, survivability
Insect bioengineers have been developing several bio-manipulation techniques for the control of insect pests and vectors. An important embodiment of bio-manipulation is transfection and transformation where foreign substances are injected into target insects that have significant implication on its genetics, pesticide susceptibility, sterility, incorporation of detectable markers that are useful for monitoring vector population, alteration of sex ratio, reduced ability to vector human and animal diseases, etc1. Microinjection offers unique advantage by incorporating precise amounts of test/ foreign substances into egg and adults with ease. It is a physical delivery system with high efficiency as compared with other manipulation techniques like chemical (endocytosis)2, mechanical (electroporation)3, viral vectors4, gene gun5, ultrasonic6 etc. However, microinjection has certain limitations as injection is a physical insult to the egg and/or adult which directly affects the viability factor of the target organism. Delivery of exogenous substances such as proteins, peptides, cDNA constructs, drugs and endosymbionts into egg and adults are the most common application of microinjection1. Microinjection methods vary to great extent to accommodate the unique physical and developmental characteristics of target insect7. Transfection (e.g. transfer of Wolbachia endosymbiotic bacteria into a different species through microinjection)8 and transformation (incorporating iRNA, cDNA for gene silencing studies in many pests and vectors)9 are the important applications of microinjection. With the recent studies aiming to incorporate live Wolbachia strains into adult mosquitoes and eggs previously not infected with Wolbachia8,10,11, the technique of microinjection has taken the centre stage. Transfection efficiency is greatly dependent on several factors such as injection volume, developmental stage of the target host insect, bleaching, desiccation, injection pressure, buffer and its pH, compatibility of the foreign substance with the target insects, post injection care, etc12. Microinjection can achieve high efficacy only by ensuring higher survivability of target insects after injection. Among several factors influencing the survivability (defined as the number of surviving insects after a span of 24 h with normal developmental characteristics indicating that they have survived the physical insult), injection by itself contributes to the survivability factor to an extent of 90 per cent as poor technique during injection relates to high mortality13. One of the major constraints for manual microinjection is poor reproducibility and inconsistency which can be trounced through improvisations. The improvised protocol during microinjection include standardization of injection volume, time, proper developmental stage, buffer temperature, resulting in higher survivability rates in embryonic and maternal microinjection. In the present study we took three important mosquito vectors of genus Aedes, Culex and Anopheles. Mosquito larvae at different time intervals and pupae were microinjected. The study was aimed to provide a common protocol of microinjection on three mosquito species with varied developmental, behavioural and evolutionary biology.
Material & Methods
The study was performed in the Department of Sericulture and Division of Biological Sciences at School of Natural Sciences, Bangalore, India.
Mosquitoes Aedes aegypti, Culex quinquefaciatus and Anopheles stephensi were field collected and reared as described previously14. Mosquito adults, pupae, larvae and embryos were used for injection. Adults were maintained in cages and fed with 8 per cent sucrose solution/raisin and blood fed with mice. Embryos were collected as described by Ansari et al15 and injected with phosphate-buffered saline (PBS) (PBS- 130 mM NaCl, 7mM Na2HPO42H2O, 3mM NaHPO4-2H2O, pH 7.0)8 and dechorionated through bleaching agent 2 per cent sodium hypochlorite16. Embryos were injected with various buffer temperature ranging from 20 to 28°C (20, 22, 24, 26 & 28°C). Microinjection apparatus consisted of an inverted microscope - Olympus CKX91, USA (for embryonic microinjection) and Stereozoom microscope Olympus SZX 10 fitted with slide holder (for larval, pupal and adult microinjection) with enough working distance for injection needles, a light source to provide illumination, fine glass injection needles from Eppendorf Femtotips II (Micropipettes), micro manipulators from Eppendorf (Transferman NK II) to position the needles mounted on an Universal Stand, Eppendorf Micro Loader to load the micropipettes. The volume was empirically calculated to be in between 0.5-1.4 pico litres based on the data17 available.
Embryonic microinjection: Blood fed Aedes and Anopheles mosquitoes after an interval of 48 h were transferred into a Drosophila culture vial with wet cotton padding and Whatman filter paper. Each vial contained around 8-10 mosquitoes (as mosquitoes lay eggs when crowded18) and placed in dark under incubation at 27°C for 45 min. Newly laid eggs were collected for embryonic microinjection. Similarly, Culex mosquitoes were blood fed overnight and newly laid egg rafts were collected in the dawn hours, 4-5 days after blood feeding by placing a cup of water containing Bermuda grass infusion, an ovipositor stimulant19. Embryos of Aedes, Culex, and Anopheles were microinjected as previously described16,20,21 with improvisations such as complete and partial bleaching, at different period intervals, different volumes and different buffer temperatures. Eggs must be supplied in abundance during microinjection. Embryonic microinjection was carried out under both Inverted and Stereozoom microscope as the contour of the mosquito eggs can be easily visualized under Stereo-zoom microscope. The buffer was filtered through 0.45 μm syringe filter (Axiva Syringe Filters, India) to avoid clogging of needle. The injection buffer (PBS) was loaded into the needle through Eppendorf micro loader or by back filling. The embryos were stuck on a double sided gum tape oriented in an anterio-posterior manner and posterior end was injected. The needle (Femtotips II, Eppendorf®) was moved during microinjection as opposed to the common practice of moving the stage, as the needle has to be moved up and down in the Z-axis to accommodate different contours of the eggs on the Z-axis. Culex, Aedes and Anopheles eggs were injected at various time intervals ranging from 15 to 90 min (Table I). Chorion shell in Anopheles eggs was physically disrupted and removed.
Table.
Effect of microinjection on different developmental stages of mosquitoes
Larval and pupal injection: Larval and pupal injection in mosquitoes was attempted to check the viability parameter of this developmental stage for microinjection. The larvae and pupae are kept in low volumes of ice cold water to slow down their rapid wriggling movements. The larvae were held against a pile of cover slips (n=5) (Fig. 1) and gently held in a forceps and injected. Care was taken that the needle penetrates enough but does not rupture the other side of the exoskeleton. The larvae and pupae were injected in the lower abdominal segments22 as in nematodes with modifications suitable for mosquito larvae and pupae (Fig. 2). The larvae and pupae were injected from the ventral side. The needle used was similar to that of the needles used for adult microinjection.
Fig. 1.
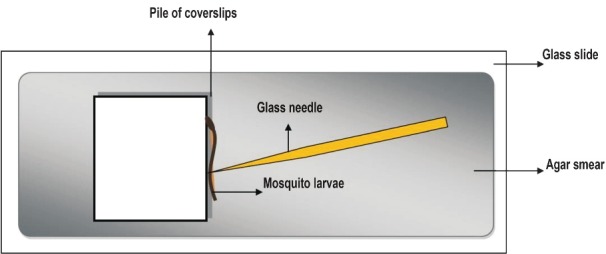
Schematic representation of larval microinjection: The larvae are laid on a slide smeared with agar to reduce its rapid movements and held against a pile of cover slips and injected in the lower abdominal segments.
Fig. 2.
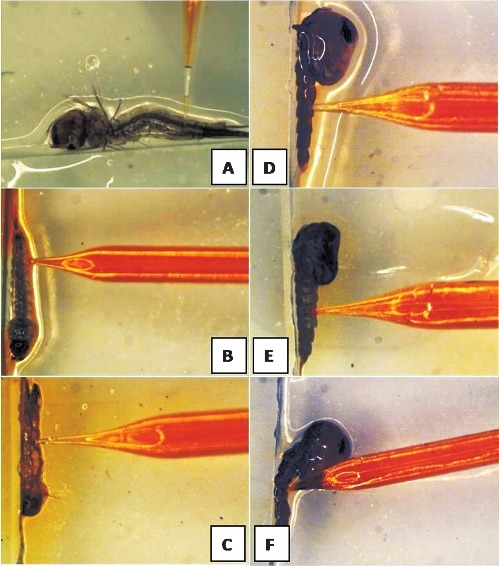
Larval and pupal microinjection: Larval microinjection of Culex, Anophleles and Aedes mosquito larvae (A-C); Pupal microinjection of Culex, Anophlelesand Aedes(D-F).
Maternal/adult microinjection: Maternal microinjection is performed using needles which were stout, less flexible, having jagged tip, with an external and internal diameter of 1.0 and 0.5 mm, respectively23. The adult mosquitoes were immobilized through placing them in CO224 hoods or exposing to lower temperature for a short duration of time and injected in the lower abdomen. Instead of common practice of sticking the wings of the adult insects on double sided gum tape18, wings were spread and stuck on the agar plate kept on ice. This would avoid the damage of wings when the mosquitoes are released back into the cages after microinjection. Adult microinjection was performed at different stages i.e. just after emergence, after fertilization, after blood meal, in starved and sucrose fed conditions. Maternal microinjection was performed under Stereozoom microscope (Olympus SZX 10) under 63X magnification. The volume injected was empirically decided based on the swelling of the abdomen. It was observed that when the needle is retrieved a bulb of the buffer oozes out of the punctured region and got back into the abdomen immediately.
Statistical analysis: The results were statistically analysed by Friedman Chi square test (χ2) for a non-parametric ‘n’ related samples for randomised block analysis of varianace.
Results
The results showed the impact of various improvisations on the enhancement of survivability factor of the adult and embryonic microinjections. The critical window period for mortality of eggs after microinjection was evaluated.
Embryos were unanimously bleached (data not shown) and it was found that partial bleaching of Aedes eggs had a higher survival rate as compared to complete bleaching. Thus during manual microinjection partial bleaching may be employed to monitor the injection of the foreign substance inside the egg shell. When embryonic microinjection was carried out at different time intervals, it was found that at 45-60 min most of the injections were successful. Of the 72 embryos injected at a time interval of 45 and 60 min, four and three Culex mosquitoes survived, respectively, indicating that Culex mosquito embryos must be injected between 45-60 min. Similarly, Anopheles eggs showed best survivability when injected as 45 min duration (8.83%) (Table). Aedes embryos were injected up to 90 min post-oviposition with a gradual decrease in the surviving embryos with the increase in time after oviposition. Aedes eggs revealed good survived when injected as 45 min followed by 60 min. Lowest ranking was observed when the eggs were injected at 15 min (χ2=5.9; P=0.32). In Culex mosquitoes highest ranking was observed in 60 min followed by 45 min (χ2=11.5; P=0.04). Anopheles mosquitoes also showed similar ranking with 60 min being the favourable followed by 45 min (χ2= 4.08; P=0.55). A lower buffer temperature of 22°C showed higher survival in Aedes mosquitoes as compared to rest of the buffer temperatures (data not shown).
In an attempt to know the critical duration after microinjection, the injected embryos were tested at regular time intervals (Fig. 3). Within the 1st h after microinjection of the 86 injected Culex embryos only 38 survived (44%). Of these 38 survivors only 28 embryos managed to overcome the 4 h period and similarly by the end of 24 h the surviving embryos from previous observation increased to 66 per cent. Similar observations were made in Aedes and Anopheles mosquitoes (data not shown). After a critical period of 24 h the survivability factor increased to 90 per cent across 3 mosquito species. A non-parametric ‘n’ related samples Friedman Chi square test (χ2) was used to evaluate the critical period after microinjection and it was found that the Friedman ranking did not change after 24 h. Based on the statistical analysis it was assumed that after the critical period of 24 h stability was regained in the biomanipulated mosquitoes (χ2=367.0; P<0.001).
Fig. 3.
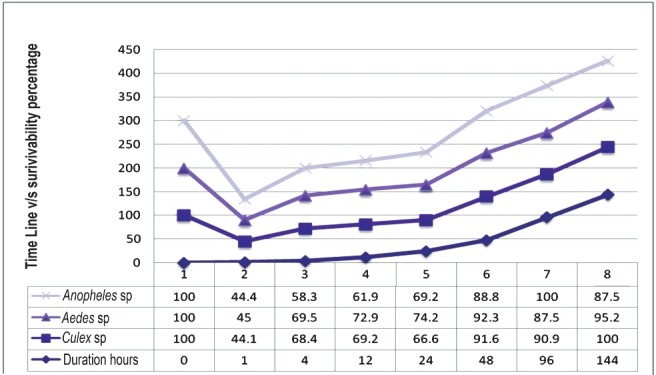
Time line showing the critical period after microinjection to calculate the survivability factor.
The injected eggs were monitored for their developmental stages, morphological appearances, keeping uninjected eggs as control for normal development. The malformed, deformed, irregularly chitinized, eggs with dents, leakages were periodically kept apart and none of these eggs hatched.
Since mosquito larvae have an aquatic habitat and are wrigglers it is hard to immobilize them for microinjection. The results showed that it was easy to manipulate the older larvae (9 days) as compared to the younger stages. Of the ‘n’ mosquito larvae injected across three different species only six survived (5.08%) of which two were from Culex, three were from Aedes and one from Anopheles (Table). Pupae normally die during microinjection and of the 55 pupae injected on the 4th and 6th day only two survived in Aedes mosquitoes (3.6%) (Table). Of the 78 pupae of Culex and Anopheles injected, none survived over of 24 h period. Of the 78 pupae of Culex and Anopheles injected, none survived over of 24 h period.
Higher survivability was observed in adults (3 days old) with an average survivability of 60 per cent across the three species of mosquitoes (Table). It was observed that older mosquitoes were poor survivors and younger mosquitoes (1 day old) are more sensitive and vulnerable to microinjection. The average percentage survivability across the three mosquito genera under different time intervals was 49 per cent which was higher as compared to embryonic microinjection (4.04%). Starving mosquitoes responded well compared to the sucrose and blood fed mosquitoes. Microinjecting blood fed mosquitoes should be avoided as it resulted in high mortality with survivability as low as 5.5 per cent in Culex, 7.1 per cent in Aedes and 0 per cent in Anopheles (Fig. 4).
Fig. 4.
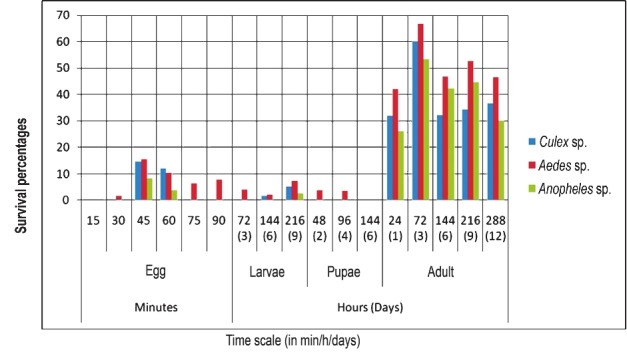
Survivability of bio-manipulated mosquitoes at different developmental stages and time intervals.
Discussion
Biomanipulation of mosquitoes is of great importance in vector control research programmes. The major constraint of microinjection is poor efficiency and reproducibility with respect to survivability of injected embryos and adults. It is observed that the larval hatch rates vary around 8 to 20 per cent using previously described methods12. The most probable cause of this variation is physical insult of embryos during injection and if the needle penetrates easily it accounts to good injection16. The number of hatching embryos can go as low as 4.8 per cent with the highest efficiency being 17.5 per cent and the survival to the adult stage gets reduced to 6.1 per cent25,27. Our study showed that the mortality factor in embryonic injection almost became zero to 5 per cent once the injected embryo passed the initial critical window period of 24 h successfully. This indicates that the mortality factor due to the technique adopted for microinjection alone contributes to mortality to an extent of 90 per cent. The results presented here allowed us to identify the critical period after microinjection. The survival efficiency of microinjected Drosophila embryos has been standardized with an efficiency of 6-8 per cent13,26,27. However, there are only a few or limited microinjection survival efficiency studies carried out in mosquito bioengineering.
The focus of the study was on the response of the target insect to the physical insult (injecting through micropipettes), and hence PBS buffer (pH 6.7) was added as the foreign substance, as it does not influence the mortality: survivability ratio28. This gives a clear picture about the mortality only due to the protocol adopted during microinjection. The results also provided an insight of the response of three mosquito species for the same protocol. A possible explanation for the variation in survivability of different mosquito species may be attributed to the difference in its developmental biology and also the variability in needle shape, volume of material injected28. Aedes mosquitoes can be injected beyond a period of 45 min up till 90 min with negligible decrease in its survivability, while Anopheles and Culex mosquitoes are best surviving when injected at 45 min after oviposition.
The present study has demonstrated the effects of bleaching, proper developmental stage for microinjection, critical period after microinjection, temperature of the foreign substance that is to be injected, adult microinjection in three different mosquito species and feeding conditions that are to be followed for adult microinjection.
The injection volume was determined empirically during injections. In accordance with earlier studies28, we assumed that the ideal injection volume would reinflate the desiccated recipient egg but not overpressurize the egg, which would result in significant cytoplasm outflow while withdrawing the needle after injection. There was significant variation in injected volumes due to differences in desiccations of recipient eggs and slight variations with respect to the mosquito species injected8. The survival rate of Culex embryos following microinjection was about 14.63 per cent9,22. The survival rate of embryos following microinjection was about 10.2 per cent indicating that despite optimizing many parameters required for microinjection significant mortality occurred. This is perhaps due to the physical insult the organism suffers during microinjection. Partial bleaching may be employed during manual microinjection to monitor the movement and volume of the foreign substance into the eggs. Microinjection at different developmental stages of mosquitoes revealed that the larval and pupal stages were difficult due to their rapid movements29. Further studies are required for developing a correct protocol to increase the survivability of larval and pupal microinjection.
Adult microinjection helps in microinjecting nucleic acid sequences enclosing a desired trait or endosymbiotic microorganisms like Wolbachia into the reproductive tract of mosquitoes before oviposition. Maternal microinjection is relatively easy than the laborious and time consuming embryonic microinjection. Maternal microinjection causes lower mortality and higher transformation frequencies than embryonic microinjection30,31. Dechorionation of eggs during microinjection acts as a significant factor in the mortality rate associated with microinjection of insect eggs31 and by adopting adult microinjection this can be avoided. Further advantages of maternal microinjection are that the microinjected eggs are incubated inside the female until oviposition which may increase the survival rate. Sucrose fed and blood fed adult mosquitoes must not be injected as it brings down the survivability factor drastically. Care must be taken to select females with eggs that are nearly, but not fully mature. Fully mature eggs burst when probed with the injection needle, leading to death of the egg and adult (female)31. With respect to the efficacy of embryonic microinjection, Aedes embryos can be easily biomanipulated followed by Culex embryos. However, Anopheles embryos are the difficult with very poor survivability during biomanipulation.
In conclusion, the present study demonstrates the important effects of bleaching, desiccation, embryonic developmental stage, immobilization method of adults, buffer temperature, etc., for microinjection on three mosquito species.
Acknowledgment
The authors are thankful to the Indian Council of Medical Research, New Delhi, for financial support.
References
- 1.Zhang Y, Yu LC. Microinjection as a tool of mechanical delivery. Curr Opin Biotechnol. 2008;19:506–10. doi: 10.1016/j.copbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Colosim A, Goncz KK, Homles AR, Kunzelmann K, Novelli G, Malone RW, et al. Transfer and expression of foreign genes in mammalian cells. Biotechniques. 2000;29:314–8. doi: 10.2144/00292rv01. [DOI] [PubMed] [Google Scholar]
- 3.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in higher electric fields. EMBO J. 1982;1:841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther W, Stein U. Viral vectors for gene transfer: A review of their use in the treatment of human diseases. Drugs. 2000;60:249–71. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lin MTS, Pulkkinen L, Uitto J, Yoon K. The gene gun: Current applications in cutanaeous gene therapy. Int J Dermatol. 2000;39:161–70. doi: 10.1046/j.1365-4362.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 6.Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophysics J. 2004;87:1013–33. doi: 10.1016/S0006-3495(03)70034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brochta DA, Atkinson PW. Transformation systems in insects. Methods Mol Biol. 2004;260:227–54. doi: 10.1385/1-59259-755-6:227. [DOI] [PubMed] [Google Scholar]
- 8.Xi Z, Dean JL, Khoo C, Dobson SL. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol. 2005;35:903–10. doi: 10.1016/j.ibmb.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen ML, O’Brochta DA, Atkinson PW, Levesque CS. Stable, germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2001;38:701–10. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- 10.Ravi Kumar H, Ramachandraswamy N, Sampath Kumar S, Prakash BM, Huchesh HC, Uday J, et al. A preliminary survey for Wolbachia and bacteriophage WO infections in Indian mosquitoes (Dipteria: Culicidae) Trop Biomed. 2010;27:384–93. [PubMed] [Google Scholar]
- 11.Rasgon JL, Xiaoxia Ren, Michael P. Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl Environ Microbiol. 2006;72:7718–22. doi: 10.1128/AEM.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo NF, Clayton JR, Fraser MJ, Kafatos FC, Collins FH. High efficiency germ-line transformation of mosquitoes. Nat Protoc. 2006;61:1312–7. doi: 10.1038/nprot.2006.221. [DOI] [PubMed] [Google Scholar]
- 13.Ringrose L. Transgenesis in Drosophila melanogaster. Methods Mol Biol. 2009;561:3–19. doi: 10.1007/978-1-60327-019-9_1. [DOI] [PubMed] [Google Scholar]
- 14.Silver JB. Mosquito ecology: field sampling methods. 3rd ed. New York: Springer Publishers; 2008. [Google Scholar]
- 15.Ansari MA, Singh KR, Brooks GD, Malhotra PR, Vaidyanathan V. The development of procedures and techniques for mass rearing of Aedes aegypti. Indian J Med Res. 1977;65:91–9. [PubMed] [Google Scholar]
- 16.Benedict Mark. Methods for Anopheles research: Microinjection methods for Anopheles embryos: Specific Anopheles techniques. Malaria Research and Reference Reagent Centre MR-4. 2011;II:1–6. [Google Scholar]
- 17.Jaffe LA, Terasaki M. Quantitative microinjection of oocytes, eggs, and embryos. Methods Cell Biol. 2004;74:219–42. doi: 10.1016/s0091-679x(04)74010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jasinskiene N, Juhn J, James AA. Microinjection of A. aegypti embryos to obtain transgenic mosquitoes. J Vis Exp. 2007;5:219. doi: 10.3791/219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millar JG, Chaney JD, Mulla MS. Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. J Am Mosq Control Assoc. 1992;8:11–7. [PubMed] [Google Scholar]
- 20.Crampton JM, Beard C, Louis C. Microinjection of mosquito embryos. In: Morris AC, editor. The molecular biology of insect disease vectors. London: Chapman and Hall; 1997. [Google Scholar]
- 21.Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, et al. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–7. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann N, Stuckas H, Lucius R, Bleiss W, Theuring F, Kalinna BH. Trans-species transfer of Wolbachia: microinjection of Wolbachia from litomosoides sigmodontis into Acanthocheilonema viteae. Parasitology. 2003;126:503–11. [PubMed] [Google Scholar]
- 23.Luna BM, Juhn J, James AA. Injection of dsRNA into female A. aegypti mosquitos. J Vis Exp. 2007;5:215. doi: 10.3791/215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terenius O, Juhn J, James AA. Injection of An. stephensi embryos to generate malaria-resistant mosquitoes. J Vis Exp. 2007;5:216. doi: 10.3791/216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh E, Mercer DR, Fu Y, Dobson SL. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Environ Microbiol. 2009;75:7783–8. doi: 10.1128/AEM.01331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–8. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 27.Brust-Mascher I, Scholey JM. Microinjection techniques for studying mitosis in the Drosophila melanogaster syncytial embryo. J Vis Exp. 2009;15:1382. doi: 10.3791/1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao Liu, Jason Pitts R, Jonathon DB, Patrick LJ, Guirong Wang, Laurence JZ. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 2010;8(8):e1000467. doi: 10.1371/journal.pbio.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blitzer EJ, Vyazunova I, Lan Q. Functional analysis of AeSCP-2 using gene expression knockdown in the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2005;14:301–7. doi: 10.1111/j.1365-2583.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller LH. Stable integration and express of a bacterial gene in the mosquito Anopheles gambia. Science. 1987;237:779–81. doi: 10.1126/science.3039658. [DOI] [PubMed] [Google Scholar]
- 31.Hoy MA, Presnail JK. Microinjection methods to transform arthropods with exogenous DNA, United States Patent, Patent No: 2001: US6,204,433 B1, 20-3-2 [Google Scholar]


