Figure 1.
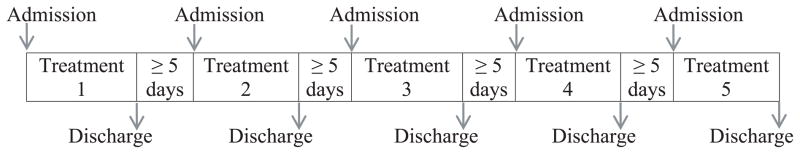
Study design. Participants (n=14) received in random order, 1 of 5 treatments: synthetic 5 mg Δ9-tetrahydrocannabinol (THC); synthetic 15 mg THC; 2 actuations of Sativex (5.4 mg THC and 5 mg cannabidiol (CBD)); 6 actuations of Sativex (16.2 mg THC + 15 mg CBD); or placebo synthetic THC and placebo Sativex.
