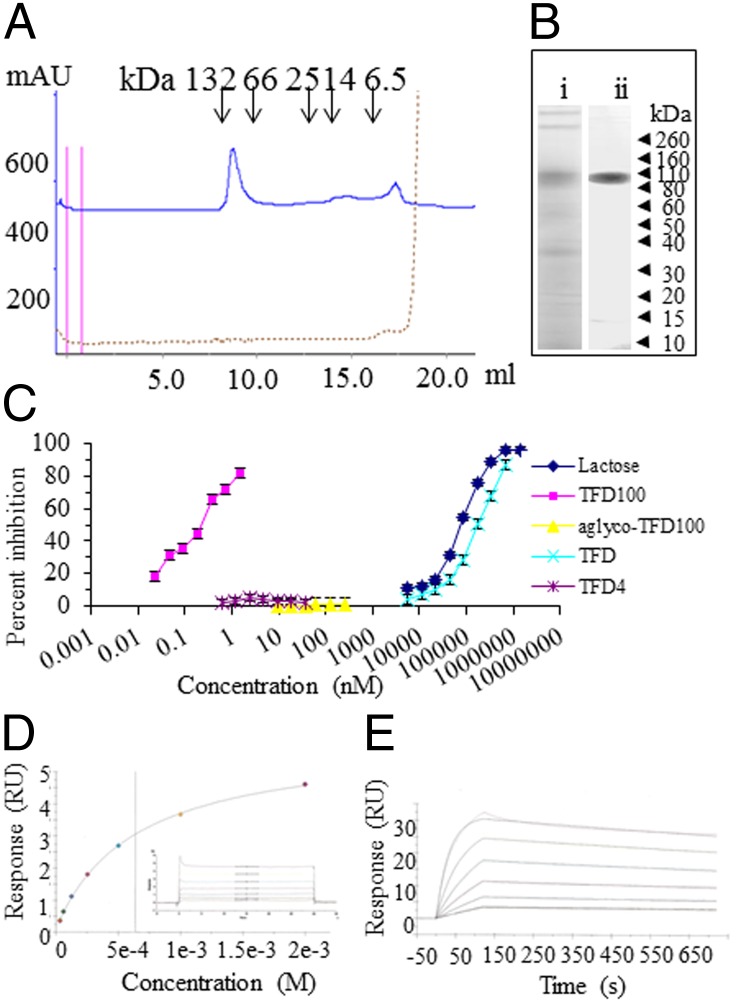
Fig. 1.
Purification and characterization of TFD100. (A) Separation of affinity-purified TFD-containing glycopeptides on a Superdex 75 10/300 GL column calibrated with BSA (dimer 132 kDa and monomer 66 kDa), chymotripsinogen (25 kDa), ribonuclease A (14 kDa), and aprotinin (6.5 kDa). (B) SDS/PAGE under reducing condition on 4–12% Bis-Tris gels followed by silver staining: (i) Crude AFGP (10 μg); (ii) TFD100 (5 μg). (C) Inhibition of gal3 binding to asialofetuin. Inhibition of gal3 binding to asialofetuin by various concentrations of compounds. (D and E) Surface plasmon resonance assay on Biacore. (D) TFD (Galβ1,3GalNAc) was measured from 31 μM to 1,000 μM, and the dissociation constant (KD) was determined to be 6.36e−4 M or 636 μM. (E) TFD100 was measured from 0.312 nM to 10 nM, and the KD was determined to be 9.677e−11 M or 97 pM. The full kinetic analysis results are as follows: ka = 3.105e+6 (1/ms); kd = 3.004e−4 (1/s); KD = 9.677e−11 M; Rmax = 31 (resonance units; RU); Tc = 2.26e+18; χ2 = 0.112 (RU2); U value = 2.