Table 3.
Population pharmacokinetic parameters of the final models using unbound and total concentrations of imatinib for the prediction of Cu (Model A) or Ctot (Model B)(simultaneous analysis)
| Total PK Parameters | Total PK parameters | Unbound PK parameters | Unbound PK parameters | ||
|---|---|---|---|---|---|
| Model A: Cu = f (Ctot)* | Model B: Ctot = f (Cu)† | ||||
| Estimate | SE (%)‡ | Estimate | SE (%)§ | ||
| CLtot (l h−1) | 13.8 | 5 | CLu (l h−1) | 386 | 6 |
| Vd,tot (l) | 409 | 17 | Vd,u (l) | 9 580 | 20 |
| katot (h−1) | 0.80 | 73 | kau (h−1) | 0.93 | 70 |
| Kd (ng ml−1) | 319 | 2 | Kd (ng ml−1) | 316 | 3 |
| L | 11 700¶ | – | L | 11 700¶ | – |
| Interindividual variability | Interindividual variability | ||||
| ωCL,tot (CV%)** | 27 | 53§ | ωCLu (CV%)** | 25 | 66§ |
| ωVd,tot (CV%)** | 48 | 75§ | ωVd,u (CV%)** | 64 | 81§ |
| Correlation CL-Vd | 0.59 | Correlation CL-Vd | 0.65 | ||
| Residual variability | Residual variability | ||||
| σ Ctot (CV%)†† | 14 | 40§ | σ Ctot (CV%)†† | 15 | 41%§ |
| σ Cu (CV%)†† | 17 | 41§ | σ Cu (CV%)†† | 14 | 41%§ |
| Correlation Cu-Ctot | 0.72 | Correlation Cu-Ctot | 0.66 | ||
In this model, total PK parameters CLtot, Vd,tot and katot are estimated and Cu are predicted using:
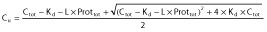 (see text).
(see text).
in this model, total unbound PK parameters are estimated CLu, Vd,u and kau, and Ctot are predicted using:
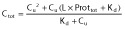 (see text).
(see text).
SE, standard error of the estimates, expressed as CV%.
SE, standard error of the variance components, taken as square (SEestimate/estimate), expressed as a percentage.
Fixed value that represent the scaling factor that accounts for the difference in concentration unit between Ctot (ng ml−1) and total protein concentration AGP (g l−1), assuming a one-to-one molar binding ratio.
Estimates of variability expressed as coefficient of variation (CV%).
Residual variability of the plasma concentration, expressed as CV%.
