Abstract
Objective
Dysphagia is one of the cardinal symptoms of Parkinson’s disease (PD). It is closely related to the quality of life and longevity of PD patients. The aim of the study is to clarify the pathophysiological mechanisms responsible for dysphagia in PD.
Design
A cross-sectional and longitudinal comparative study.
Setting
Tohoku University Hospital.
Participants
Eight patients with dysphagia, 15 patients without dysphagia and 10 normal control subjects.
Main outcome measures
The time needed for swallowing initiation and changes in brain glucose metabolism at baseline and after a 3-year follow-up period.
Results
The time needed for swallowing initiation was significantly longer in the patients with dysphagia compared with the patients without dysphagia at baseline and after the 3-year follow-up period (p<0.05). The patients with dysphagia exhibited hypometabolism in the supplementary motor area (SMA) and the anterior cingulate cortex (ACC) compared with the 10 normal control subjects at baseline (uncorrected p<0.001). After the 3-year follow-up period, the number of brain areas showing hypometabolism increased, involving not only the SMA and the ACC but also the bilateral medial frontal lobes, middle cingulate cortex, thalamus and right superior, middle, inferior and orbital frontal gyri (uncorrected p<0.001). In contrast, the patients without dysphagia showed virtually no regional hypometabolism at baseline (uncorrected p<0.001) and only a small degree of hypometabolism in the SMA and ACC after the 3-year follow-up period (uncorrected p<0.001).
Conclusions
These results suggest that dysphagia in PD patients is mainly related to a difficulty in swallowing initiation that is based on a combination of poor movement planning due to SMA dysfunction and impaired cognitive processing due to ACC dysfunction.
Keywords: Parkinson's Disease, Dysphagia, PET
Article summary.
Article focus
Cortical hypometabolism associated with dysphagia in Parkinson's disease (PD) was statistically examined at baseline and after a 3-year follow-up period.
Key messages
The multiple cortical impairments, mainly in the supplementary motor area (SMA) and anterior cingulate cortex (ACC), might be responsible for the dysphagia in PD.
The time needed for swallowing initiation was significantly longer in patients with dysphagia.
Dysphagia in PD patients is mainly related to a difficulty in swallowing initiation that is based on a combination of poor movement planning owing to SMA dysfunction and impaired cognitive processing due to ACC dysfunction.
Strengths and limitations of this study
The strength of this study is that it is the first to statistically examine the associations between cortical hypometabolism and dysphagia in PD patients as a cross-sectional and longitudinal comparative study.
A limitation is that the findings may not be related to dysphagia alone because 18F-fluorodeoxyglucose-positron emission tomography cannot be used for dynamic scanning during swallowing. Another weakness of this study is the absence of videofluoroscopy as a swallowing evaluation.
Introduction
Parkinson's disease (PD) is primarily characterised by motor dysfunctions, some of which, such as tremor, rigidity and bradykinesia, respond well to dopamine replacement therapy, whereas others, such as postural instability, dysarthria and dysphagia, remain intractable and often impair the quality of life in advanced cases. The involvement of non-dopaminergic systems is implied in such dopamine-refractory symptoms; however, the detailed pathophysiological mechanisms of these systems are still elusive. In particular, swallowing difficulty is directly associated with malnutrition and difficulty in drug taking; moreover, this symptom sometimes results in aspiration pneumonia, the main cause of death in PD patients.1–3 The voluntary transport of food through the oral cavity, pharynx and oesophagus to the stomach requires sequential motor events. Deglutition occurs through five consecutive phases: anticipatory (cognitive), preparatory (masticatory), oral, pharyngeal and oesophageal. Dysphagia is associated with all of these stages.4–8
In general, dysphagia in PD is thought to reflect impaired function of the medullary swallowing centre.9 The involvement of the higher central nervous system areas is also implied, but remains to be elucidated. In the present study, we investigated the swallowing functions of PD patients and compared them with the changes in the cortical metabolism using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Moreover, we investigated clinical and imaging data not only at baseline, but also after a 3-year follow-up period, making this research a longitudinal study.
Methods
Participants
All of the 43 PD patients were diagnosed based on the UK PD Brain Bank criteria for idiopathic PD.10 The extent of dementia was evaluated in all of the indivuduals, using the clinical dementia rating (CDR). PD patients with a CDR score greater than 0.5 were excluded to minimise artefacts related to cognitive impairment. We defined a score of 0 as no dysphagia and a score of >1 as dysphagia, according to part II of the unified Parkinson's disease rating scale (UPDRS). The UPDRS total scores, Mini-Mental State Examination (MMSE), swallowing function and PET studies were evaluated during the ‘on’ state, that is, with the administration of antiparkinsonian drugs without L-dopa-induced dyskinesia. Ten age-matched control subjects (4 women and 6 men; mean age 64.4±4.12 years; mean MMSE score 28.7±1.49) were collected to compare with PD patients with and without dysphagia for PET analysis. All of the procedures were approved by the Ethical Committee of the Tohoku University Graduate School of Medicine. Written informed consent was obtained from each individual after a full explanation of the entire 3- year longitudinal study.
PET procedure
The FDG-PET scans were performed in all 23 PD patients and 10 age-matched control subjects. All of the individuals fasted for at least 5 h prior to PET scanning. To minimise the effects of external stimuli during a 1 h FDG uptake period after intravenous injection of 185–218 MBq FDG, the individuals stayed in a quiet room wearing an eye mask under resting conditions. PET scans were acquired for 10 min under resting conditions. Dynamic PET scans were taken in three-dimensional mode using a BiographDuo PET scanner (Siemens Medical Systems, Inc, Iselin, New Jersey, USA). The in-plane and axial resolutions of the scanner were 3.38 and 3.38 mm, respectively. Attenuation correction was performed with a CT scan. Image reconstructions were performed using ordered subset expectation maximisation algorithms (16 subsets × 6 iterations) using a Gaussian filter with full-width at half-maximum = 2.0 mm in a 256×256 matrix, with a pixel size of 1.33×1.33 mm, a slice thickness of 2.0 mm and a field of view of 340 mm. The FDG-PET scans acquired at the follow-up were performed in an identical manner.
Data analysis
Statistical parametric mapping (SPM) was used for group comparisons of PET images. First, all of the PET images were spatially normalised with linear and non-linear parameters using SPM2 software (Welcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB (The MathWorks, Inc, Sherborn, Maryland, USA). A three-dimensional Gaussian filter of 10 mm was used to smooth each image. Global normalisation was performed using SPM's ‘proportional scaling’, and proportional threshold masking was set at 0.8. Next, the regional metabolic abnormalities in the PD patients with and without dysphagia at baseline and after the 3-year follow-up period were estimated by comparison with 10 age-matched control subjects using proportional scaling. The statistical threshold was p<0.001 (uncorrected) with an extent threshold of 40 voxels.
Evaluation of swallowing
We evaluated the time needed for swallowing initiation and the 30 s swallowing frequency in all 23 PD patients and 10 healthy volunteers. The index and ring finger pads of the investigator were placed on the thyroid cartilage and laryngeal prominence. The time needed for swallowing initiation was defined as the time until movement of the thyroid cartilage and laryngeal prominence following the verbal guidance cue to begin swallowing using a timer. The time of swallowing initiation across three trials was averaged. We also measured the swallowing frequency by asking the patients to swallow as many times as possible in 30 s and counting the movements of the thyroid cartilage and laryngeal prominence.11 12
The patient profiles were statistically analysed using two-sample t tests. For the swallowing function, the within-group differences of the patients with and without dysphagia were assessed using paired t tests.
Results
Of the 43 potentially eligible cases, 16 were excluded from the analysis because of dementia. The 27 patients who fulfilled the above entry criteria included 9 patients with dysphagia and 18 patients without dysphagia. The mean (±SD) age was 68.2±3.23 years in patients with dysphagia and 65.8±3.94 years in patients without dysphagia. All of the patients were right-handed. Eight of 9 PD patients with dysphagia and 15 of 18 patients without dysphagia at baseline participated in the 3-year follow-up study. The details of the excluded patients were as follows: 2 patients without dysphagia went to other hospitals and 1 patient without dysphagia and 1 patient with dysphagia were unable to be evaluated during the follow-up period because of the introduction of a feeding tube (figure 1).
Figure 1.
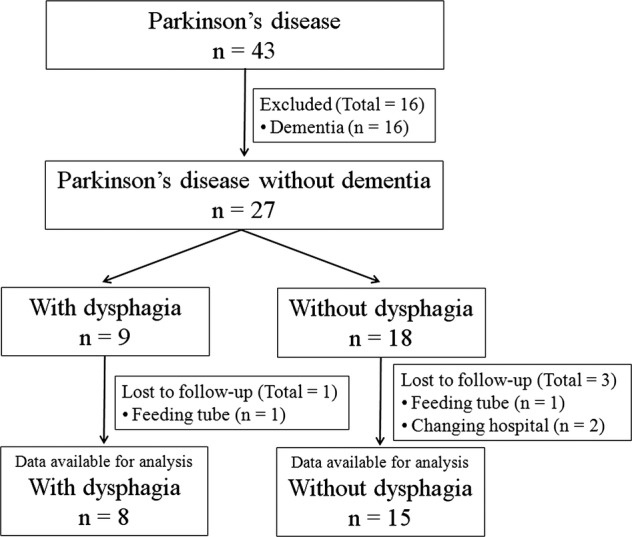
Parkinson's disease patients and follow-up flow diagram.
Clinical features and medications were summarised in table 1. No significant differences were found in the age, Hoehn-Yahr stage and dosage of antiparkinsonian agents, including L-dopa (table 1) or 30 s swallowing frequency (figure 2B), but significant differences were found between the two groups in disease duration, UPDRS motor score and time needed for swallowing initiation at baseline (p<0.05, table 1 and figure 2A). After the 3-year follow-up period, the differences in the time needed for swallowing initiation were still clear, but no significant differences became evident in the UPDRS motor scores (table 1 and figure 2A). Significant differences were revealed in the swallowing frequency within 30 s between the two groups (p<0.05, table 1 and figure 2B). The times needed for swallowing initiation (1.02±0.36 s) and the 30 s swallowing frequency (5.10±2.42) in 10 healthy volunteers were almost the same as those of PD without dysphagia. There was no significant difference in the L-dopa equivalent dose between baseline and 3-year follow-up in PD with dysphagia using paired t test (p>0.05), while a significant difference was found in PD without dysphagia (p<0.05). No significant differences were found in the UPDRS motor score between baseline and 3-year follow-up within groups using paired t tests (p>0.05).
Table 1.
Profiles of PD patients
| Baseline |
Follow-up |
|||
|---|---|---|---|---|
| Parameters | With dysphagia | Without dysphagia | With dysphagia | Without dysphagia |
| Number of patients | 8 | 15 | ||
| Sex (M/W) | 6/ 2 | 4 /11 | ||
| Age (years) | ||||
| Mean | 67.8±3.11 | 65.2±4.02 | ||
| Range | 63–71 | 60–74 | ||
| Disease duration (years) | 6.75±3.73* | 3.53±3.58 | ||
| MMSE | 28.5±1.93 | 28.5±1.51 | 27.9 ± 2.80 | 28.4 ± 1.60 |
| Hoehn-Yahr stage | 2.75±0.27 | 2.37±0.67 | 3.06 ± 0.42 | 2.93 ± 0.53 |
| UPDRS motor score | 23.4±7.05* | 14.8±7.49 | 21.0 ± 5.93 | 17.4 ± 11.1 |
| L-dopa equivalent dose (mg/day) | 400±311 | 267±321 | 590±155 | 514±311 |
Values are mean±SD or the number of patients.
*Significant difference p<0.05 between with and without dysphagia.
M, men; MMSE, Mini-Mental State Examination; PD, Parkinson's disease; UPDRS, unified Parkinson's disease rating scale; W, women.
Figure 2.
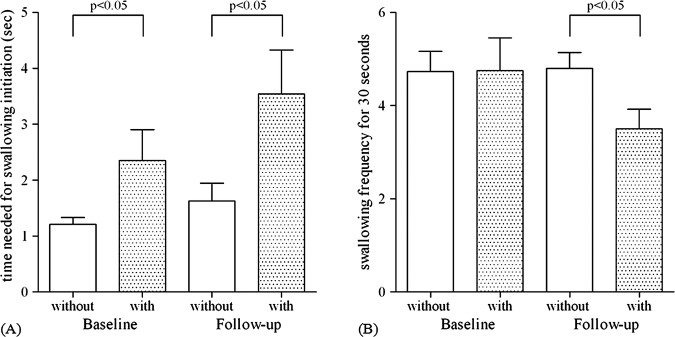
The time needed for swallowing initiation and the 30 s swallowing frequency in Parkinson's disease (PD) patients with and without dysphagia at baseline and after a 3-year follow-up period. There were significant differences between PD patients with and without dysphagia in the time needed for swallowing initiation at baseline and after a 3-year follow-up period (p<0.05) (A). In the 30 s swallowing frequency, there was no significant difference between PD patients with and without dysphagia at baseline (p>0.05), but a significant difference between the two groups was evident after the 3-year follow-up period (p<0.05) (B).
A comparison of the regional cerebral glucose of PD patients with that of normal controls demonstrated hypometabolism in the supplementary motor area (SMA) and the anterior cingulate cortex (ACC) in patients with dysphagia at baseline (uncorrected p<0.001, figure 3A). These metabolic changes showed no significant correlations with the severity of the Hoehn-Yahr stages (uncorrected p<0.001, data not shown) or the UPDRS motor scores (uncorrected p<0.001, data not shown). Furthermore, no regional hypermetabolism was found in PD patients with dysphagia compared with normal control subjects (uncorrected p<0.001, data not shown). Additionally, no relationships were observed between the changes in the regional cerebral glucose metabolism and the doses of antiparkinsonian agents, including L-dopa (p>0.05, data not shown). After the 3-year follow-up period, the areas of hypometabolism included not only the SMA and the ACC but also the bilateral medial frontal lobes, middle cingulate cortex, thalamus and right superior, middle, inferior and orbital frontal gyri (uncorrected p<0.001, figure 3B). Only a small degree of hypermetabolism in the left middle and right superior occipital lobes, left middle temporal lobe, left supramarginal gyrus and left calcarine cortex was found in PD patients with dysphagia compared to the normal control subjects (uncorrected p<0.001, data not shown). In contrast, PD patients without dysphagia showed virtually no regional hypometabolism at baseline (uncorrected p<0.001, figure 4A) and only a small degree of hypometabolism in the SMA and ACC after the 3-year follow-up period (uncorrected p<0.001, figure 4B) compared with normal controls. On the other hand, in PD patients without dysphagia, no regional hypermetabolism was found at baseline (uncorrected p<0.001, data not shown) and only a small degree of hypermetabolism in the left supramarginal gyrus, left postcentral gyrus, and left middle and superior lobes was found after the 3-year follow-up period (uncorrected p<0.001, data not shown). Medullary hypometabolism was not found either at baseline or after a 3-year follow-up period (uncorrected p<0.001, figures 3 and 4).
Figure 3.
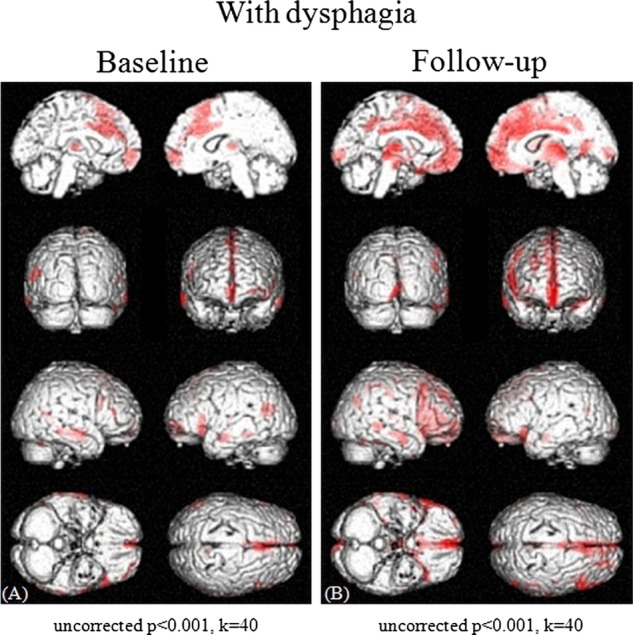
Cross-sectional analyses of brain maps showing the differences between Parkinson's disease (PD) patients with dysphagia and normal control subjects at baseline (A) and after a 3-year follow-up period (B). Various areas showed differences in the standardised PET data, and areas with a height threshold of p<0.001 (uncorrected) and an extent threshold of 40 voxels are illustrated. A comparison of regional cerebral glucose metabolism values demonstrated hypometabolism in the SMA and ACC in the PD patients with dysphagia compared with normal control subjects at baseline (uncorrected p<0.001, threshold=40 voxels) (A). After a 3-year follow-up period, the areas of hypometabolism included not only the SMA and the ACC but also the bilateral medial frontal lobes, middle cingulate cortex, thalamus and right superior, middle, inferior and orbital frontal gyri (uncorrected p<0.001, threshold=40 voxels) (B).
Figure 4.
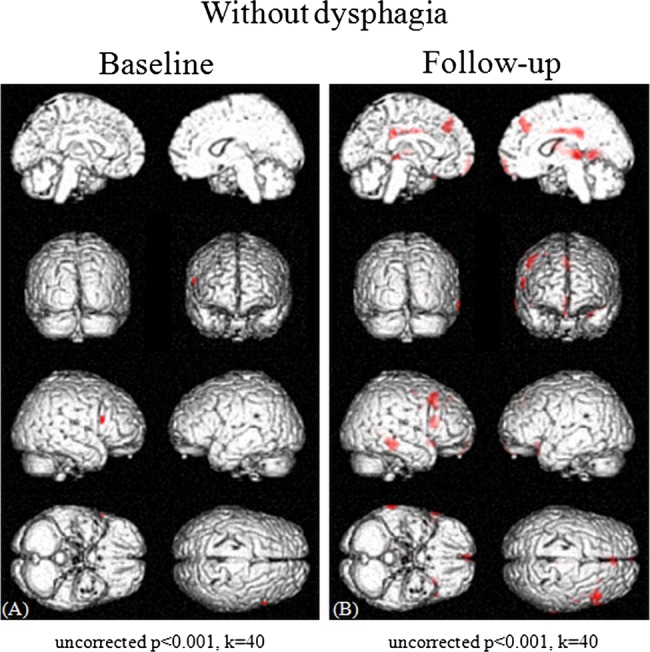
Cross-sectional analyses of brain maps showing differences between PD patients without dysphagia and normal control subjects at baseline (A) and after a 3-year follow-up period (B). Various areas showed differences in the standardised PET data, and areas with a height threshold of p<0.001 (uncorrected) and an extent threshold of 40 voxels are illustrated. The PD patients without dysphagia showed virtually no hypometabolism at baseline (uncorrected p<0.001, threshold=40 voxels) (A) and only a small degree of hypometabolism in the SMA and ACC after a 3-year follow-up period (uncorrected p<0.001, threshold=40 voxels) (B) compared with normal control subjects.
Discussion
The present results suggested that although several motor cortical areas control deglutition, multiple cortical impairments, mainly in the SMA and ACC, might be responsible for dysphagia in PD. These results were in agreement with the findings of previous activation studies such as functional MRI13–18 and PET19 20 of normal deglutition. Impairments in these areas did not appear to be closely associated with the degree of general motor dysfunction and cognitive impairments, as no significant correlations were found between the UPDRS motor scores or the MMSE scores and the degree of hypometabolism in these areas (uncorrected p<0.001, data not shown). Moreover, although there were no significant changes in the UPDRS motor scores and the MMSE scores, except for the L-dopa equivalent dose in PD without dysphagia, between baseline and 3-year follow-up in each group (table 1), cortical hypometabolism in the medial frontal lobes was markedly extended, especially in cases with dysphagia (figure 3). Interestingly, in patients with dysphagia, both of the swallowing indices showed tendencies toward exacerbation during the 3-year follow-up period, although the differences were not significant (figure 2). Although no significant difference was found in the UPDRS motor score between PD with and without dysphagia after a 3-year follow-up, the time needed for swallowing initiation was worsening in PD with dysphagia. Bradykinesia did not appear to be directly related to the outcome measurement results for evaluation of swallowing. We could not find any compensatory mechanisms for dysphagia because regional hypermetabolism was not found in PD with and without dysphagia at baseline.
Major subcortical inputs to the SMA via the thalamus arise from the globus pallidus and the substantia nigra.21 The SMA also receives limbic inputs from the cingulate cortex.22 The SMA is known to be important in mediating and preparing complex sequences of movement23 and in movement planning and execution.24–28 PET and functional MRI studies demonstrated SMA activation during the swallowing task.19 29 Activation of the SMA preceding the onset of volitional swallowing can also be demonstrated by the assessment of the Bereitschaftspotential, one of the premotor potentials that is considered to reflect the activities of the SMA.30 The amplitude of the Bereitschaftspotential31 was shown to be significantly lower in PD patients compared with age-matched controls.32 PET and single-photon emission CT studies also demonstrated dysfunction of the SMA in PD.33–37 Therefore, the SMA dysfunction in PD patients with dysphagia is likely to be related to the impaired volitional initiation of swallowing.
The ACC is connected to both the insula38 and the amygdalae,39 which are implicated in autonomic function and somatosensory and viscerosensory input. Intense stimulation of the oesophagus8 40 and changes in gastrointestinal motility39 were shown to activate the ACC. The ACC is thought to play an important role in receiving sensory stimuli from the alimentary tract. The functions of the rostral ACC are autonomic regulation and visceromotor control.14 In fact, the rostral ACC is activated during automatic swallowing.14 In contrast, the more dorsal and caudal regions of the ACC function in skeletomotor control, including movement regulation and premotor function, response selection, attention to willed action and nociception.26 The intermediate and caudal regions of the ACC are activated during voluntary swallowing.14 18 41 As PD patients with dysphagia showed hypometabolism in the intermediate and caudal regions of the ACC, corresponding to Brodmann’s area 24 as shown in figure 3A, the dysphagia in PD patients appears to be associated with difficulties in the processing of voluntary swallowing. In fact, the mean time needed to initiate swallowing was longer in cases with dysphagia (figure 2).
In this study, the time needed for swallowing initiation and swallowing frequency for 30 s showed high reproducibility. The time needed for swallowing initiation was thought to reflect the time from the anticipatory to the pharyngeal stages of deglutition, but other stages were not evaluated. Additional evaluation, such as videofluoroscopy, is needed to understand the relationship between brain hypometabolism and dysphagia in PD patients. Interestingly, in 4 of 8 patients with dysphagia, the time needed for swallowing initiation was above average (2.35 s), and, in fact, percutaneous endoscopic gastrostomies were performed in 2 of these 4 patients within 4 years of the baseline study. Thus, by measuring the time needed for swallowing initiation, we may be able to predict the long-term prognosis of swallowing difficulty.
Conclusion
In conclusion, the data presented in this study suggested that dysphagia in PD patients was related to dysfunctions in the SMA and ACC, resulting in poor movement planning of voluntary swallowing. The results also suggest that training exercises, which can activate broader cortical areas including the SMA and ACC,17 may be useful to alleviate swallowing difficulty.
Supplementary Material
Footnotes
Contributors: AK and AT were the main researchers of the study. All authors helped to plan the study, analyse the data and critically revise successive drafts of the manuscript, and they also approved the final version of the manuscript.
Funding: This study was supported by a grant for a Symposium on Catecholamine and Neurological Disorders and a Grant-in-Aid for Scientific Research (C) (23591266) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethical Committee of Tohoku University Graduate School of Medicine.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Nakashima K, Maeda M, Tabata M, et al. Prognosis of Parkinson's disease in Japan. Tottori University Parkinson's Disease Epidemiology (TUPDE) Study Group. Eur Neurol 1997;38(Suppl 2):60–3 [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Morris JG, Traficante R, et al. The sydney multicentre study of Parkinson's disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry 1999;67:300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fall PA, Saleh A, Fredrickson M, et al. Survival time, mortality, and cause of death in elderly patients with Parkinson's disease: a 9-year follow-up. Mov Disord 2003;18:1312–16 [DOI] [PubMed] [Google Scholar]
- 4.Bushmann M, Dobmeyer SM, Leeker L, et al. Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology 1989;39:1309–14 [DOI] [PubMed] [Google Scholar]
- 5.Bird MR, Woodward MC, Gibson EM, et al. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in 16 patients. Age Ageing 1994;23:251–4 [DOI] [PubMed] [Google Scholar]
- 6.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson's disease. Dysphagia 1996;11:14–22 [DOI] [PubMed] [Google Scholar]
- 7.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson's disease. Dysphagia 1997;12:11–18; discussion 9–20 [DOI] [PubMed] [Google Scholar]
- 8.Aziz Q, Andersson JL, Valind S, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology 1997;113:50–9 [DOI] [PubMed] [Google Scholar]
- 9.Hunter PC, Crameri J, Austin S, et al. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry 1997;63:579–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oguchi K, Saitoh E, Mizuno M, et al. The repetitive saliva swallowing test (RSST) as a screening test of functional dysphagia (1) normal values of RSST. Jpn J Rehabil Med 2000;37:375–82 [Google Scholar]
- 12.Tamura F, Mizukami M, Ayano R, et al. Analysis of feeding function and jaw stability in bedridden elderly. Dysphagia 2002;17:235–41 [DOI] [PubMed] [Google Scholar]
- 13.Kern MK, Jaradeh S, Arndorfer RC, et al. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 2001;280:G354–60 [DOI] [PubMed] [Google Scholar]
- 14.Martin RE, Goodyear BG, Gati JS, et al. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 2001;85:938–50 [DOI] [PubMed] [Google Scholar]
- 15.Martin R, Barr A, MacIntosh B, et al. Cerebral cortical processing of swallowing in older adults. Exp Brain Res 2007;176:12–22 [DOI] [PubMed] [Google Scholar]
- 16.Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 2001;140:280–9 [DOI] [PubMed] [Google Scholar]
- 17.Martin RE, MacIntosh BJ, Smith RC, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol 2004;92:2428–43 [DOI] [PubMed] [Google Scholar]
- 18.Toogood JA, Barr AM, Stevens TK, et al. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res 2005;161:81–90 [DOI] [PubMed] [Google Scholar]
- 19.Hamdy S, Rothwell JC, Brooks DJ, et al. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol 1999;81:1917–26 [DOI] [PubMed] [Google Scholar]
- 20.Harris ML, Julyan P, Kulkarni B, et al. Mapping metabolic brain activation during human volitional swallowing: a positron emission tomography study using [18F]fluorodeoxyglucose. J Cereb Blood Flow Metab 2005;25:520–6 [DOI] [PubMed] [Google Scholar]
- 21.Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science 1993;259:819–21 [DOI] [PubMed] [Google Scholar]
- 22.Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res 1994;19:251–68 [DOI] [PubMed] [Google Scholar]
- 23.Fried I, Katz A, McCarthy G, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci 1991;11:3656–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 1991;11:667–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 2002;77:677–82 [DOI] [PubMed] [Google Scholar]
- 26.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118(Pt 1):279–306 [DOI] [PubMed] [Google Scholar]
- 27.Deiber MP, Ibanez V, Sadato N, et al. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 1996;75:233–47 [DOI] [PubMed] [Google Scholar]
- 28.Richter W, Andersen PM, Georgopoulos AP, et al. Sequential activity in human motor areas during a delayed cued finger movement task studied by time-resolved fMRI. Neuroreport 1997;8:1257–61 [DOI] [PubMed] [Google Scholar]
- 29.Mosier K, Patel R, Liu WC, et al. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 1999;109:1417–23 [DOI] [PubMed] [Google Scholar]
- 30.Huckabee ML, Deecke L, Cannito MP, et al. Cortical control mechanisms in volitional swallowing: the Bereitschaftspotential. Brain Topogr 2003;16:3–17 [DOI] [PubMed] [Google Scholar]
- 31.Kornhuber HH, Deecke L. Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials.. Pflugers Arch Gesamte Physiol Menschen Tiere 1965;284:1–17 [PubMed] [Google Scholar]
- 32.Dick JP, Rothwell JC, Day BL, et al. The Bereitschaftspotential is abnormal in Parkinson's disease. Brain 1989;112(Pt 1):233–44 [DOI] [PubMed] [Google Scholar]
- 33.Playford ED, Jenkins IH, Passingham RE, et al. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol 1992;32:151–61 [DOI] [PubMed] [Google Scholar]
- 34.Grafton ST, Waters C, Sutton J, et al. Pallidotomy increases activity of motor association cortex in Parkinson's disease: a positron emission tomographic study. Ann Neurol 1995;37:776–83 [DOI] [PubMed] [Google Scholar]
- 35.Jahanshahi M, Jenkins IH, Brown RG, et al. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain 1995;118 (Pt 4):913–33 [DOI] [PubMed] [Google Scholar]
- 36.Brooks DJ. PET and SPECT studies in Parkinson's disease. Baillieres Clin Neurol 1997;6:69–87 [PubMed] [Google Scholar]
- 37.Kikuchi A, Takeda A, Kimpara T, et al. Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson's disease. J Neurol Sci 2001;193:29–36 [DOI] [PubMed] [Google Scholar]
- 38.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol 1982;212:38–52 [DOI] [PubMed] [Google Scholar]
- 39.Pandya DN, Van Hoesen GW, Domesick VB. A cingulo-amygdaloid projection in the rhesus monkey. Brain Res 1973;61:369–73 [DOI] [PubMed] [Google Scholar]
- 40.Kern MK, Birn RM, Jaradeh S, et al. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology 1998;115:1353–62 [DOI] [PubMed] [Google Scholar]
- 41.Paus T, Otaky N, Caramanos Z, et al. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 1996;376:664–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


