Abstract
Dysregulation of pain neurocircuitry and neurochemistry has been increasingly recognized as playing a critical role in a diverse spectrum of diseases including migraine, fibromyalgia, depression, and PTSD. Evidence presented here supports the hypothesis that alcohol dependence is among the pathologies arising from aberrant neurobiological substrates of pain. In this review, we explore the possible influence of alcohol analgesia and hyperalgesia in promoting alcohol misuse and dependence. We examine evidence that neuroanatomical sites involved in the negative emotional states of alcohol dependence also play an important role in pain transmission and may be functionally altered under chronic pain conditions. We also consider possible genetic links between pain transmission and alcohol dependence. We propose an allostatic load model in which episodes of alcohol intoxication and withdrawal, traumatic stressors, and injury are each capable of dysregulating an overlapping set of neural substrates to engender sensory and affective pain states that are integral to alcohol dependence and comorbid conditions such as anxiety, depression, and chronic pain.
Keywords: Alcohol dependence, Chronic pain, Nociception, Negative affect, Stress, Anxiety, Allostasis
1. Introduction
Chronic pain affects an estimated 116 million American adults and costs the nation up to $635 billion each year (Committee on Advancing Pain Research, Care, and Education; Institute of Medicine, 2011). Approximately 18 million Americans suffer from alcohol abuse or dependence, contributing to 100,000 deaths and $185 billion in costs annually (Grant et al., 2004a). Although the relationship between pain and opiate misuse has been extensively studied, considerably less attention has been devoted to the study of pain and alcohol use despite evidence that alcohol ingestion can acutely reduce pain. In addition, associations between chronic pain conditions and alcohol problems have been reported with episodes of alcohol abuse antedating chronic pain in some people and alcohol dependence emerging after the onset of chronic pain in others (Katon et al., 1985). In light of the great public health impact of both alcohol dependence and chronic pain, a mechanistic understanding of this relationship is important for preventing and treating both problems.
In addition to the well-established acute alcohol actions (reward, stimulation, and impairment) and withdrawal effects (CNS/ANS hyperexcitability, anxiety, sleep disturbances, and dysphoria) that contribute to excessive drinking and relapse, alcohol produces analgesia followed by hyperalgesia after withdrawal (Gatch, 2009). We hypothesize here that pain sensitivity, analgesic actions of alcohol and withdrawal-induced hyperalgesia also contribute to alcohol misuse and alcohol addiction. This hypothesis is supported by observations that problem drinkers are more likely to report pain conditions and heightened sensitivity to painful stimulation than the general population. Alcohol dependence also was found to be a major predictor of pain severity following serious injury (Castillo et al., 2006; Holmes et al., 2010). Other studies suggest that people who do not have drinking problems, but have a positive family history of alcoholism (FHP), are more sensitive to painful stimulation than those having no family history of alcoholism (FHN; Stewart et al., 1995). Likewise, people with chronic pain conditions are more likely to have family members with drinking problems (Goldberg et al., 1999; Katon et al., 1985). Another facet of this relationship is revealed in studies showing that people experiencing chronic pain turn to alcohol presumably for relief (e.g., Brennan et al., 2005; Riley and King, 2009).
An important aspect of the pain-alcohol link rests on neural substrates of alcohol reinforcement and dependence which are also observed to influence critical pain transmission and perception functions. In this review, we discuss neuroreceptor systems that may play a dual role in alcohol reinforcement and pain transmission and the implications this has for imparting genetic vulnerability to develop pain-related problems in addition to compulsive alcohol seeking. We also extend previous thinking in which alcohol dependence and addiction are thought to arise when brain reward and extrahypothalamic stress systems, including the central nucleus of the amygdala (CeA), are dysregulated by chronic alcohol intoxication and withdrawal cycles such that there is a progressive and enduring shift away from a homeostatic reward set point seemingly maintained in recreational drinkers (Edwards and Koob, 2010; Koob and Le Moal, 2001). The dysregulated reward and stress systems result in the emergence of allostasis-like negative emotional states during abstinence periods. According to this view, alcohol is consumed in ever increasing amounts to alleviate negative motivational symptoms arising in the absence of alcohol. Below we suggest affective as well as sensory dimensions of pain should be considered as part of the abstinence syndrome contributing to alcohol use. In addition, we propose a model in which pain-related affective and sensory dysregulation associated with functional changes in an overlapping set of neurocircuitry occurs in response to repeated episodes of alcohol intoxication and withdrawal, unresolved chronic pain, stress and other insults. The hypothesized model helps explain the relationship between pain symptoms and alcohol use, as well as comorbidity between alcohol dependence, anxiety disorders, and chronic pain. A mechanistic understanding of how these core circuits are dysregulated by diverse insults will promote strategies for preventing and treating a spectrum of debilitating pain-associated disorders.
2. Analgesia, hyperalgesia and alcohol dependence
2.1. Alcohol analgesia
Alcohol dose-dependently produces analgesia in humans and animals and the possibility that analgesia may contribute to alcohol use is supported by reports that as many as 25% of people experiencing pain self-medicate with alcohol (e.g., Riley and King, 2009). Pseudoaddiction describes an addictive-like seeking of analgesic drugs by people experiencing chronic pain in an effort to alleviate undertreated pain (Weissman and Haddox, 1989). Although pseudoaddiction is primarily associated with opiates, understanding conditions under which alcohol significantly reduces pain may identify people at risk for engaging in harmful drinking in an attempt to alleviate pain.
Alcohol’s pain alleviating effects are associated with vulnerability for becoming alcohol dependent. In general, excessive alcohol users and FHP individuals are more sensitive to painful stimulation and are more responsive to pain-reducing effects of alcohol than control subjects to the extent that alcohol normalizes pain and discomfort perceptions (Stewart et al., 1995). In alcoholics, heightened alcohol analgesia may also arise from extensive experience through which they come to expect that alcohol alleviates pain. For example, bourbon whiskey reduced pain perception by alcoholic men, but had no analgesic effect in non-alcoholic subjects (Cutter et al., 1976). Subsequent research in male university students (Brown and Cutter, 1977) showed that alcohol’s ability to reduce pain was influenced by drinking experiences such that customary bar drinkers and subjects with a history of sickness after drinking experienced greater pain reduction after drinking alcohol than those who drank with families or who had no history of drinking-related sickness. The maximum level of pain reduction was associated with the subject’s preferred alcohol dose.
Tolerance develops to alcohol’s analgesic effects with repeated exposure through physiological mechanisms that include learning mechanisms. When alcohol is administered to rats in a liquid diet for 10 days, analgesic effects peak within 2–4 days and subside with continued administration until pain responses return to baseline levels by day 10 (Gatch, 2009). Such tolerance can be environment-dependent, as exposure to alcohol in itself was not sufficient to produce tolerance to alcohol analgesia, i.e., tolerance was observed only in animals receiving repeated pain assessments during alcohol administration, but not in animals which did not receive analgesia tests during the alcohol exposure period (Jørgensen and Hole, 1984). Other studies suggest environment-independent tolerance. For example, rats receiving alcohol injections in a distinct environment developed tolerance to alcohol’s analgesic effects regardless of whether they also received tail-flick tests in the same environment (Tiffany et al., 1987). Learning mechanisms do not appear to influence tolerance development when rats receive alcohol in a liquid diet, however, because comparable tolerance effects were observed regardless of whether repeated pain tests were given during alcohol administration (Gatch and Lal, 1999). These studies suggest that conditions for developing tolerance to alcohol analgesia vary, and that experiencing sustained or exaggerated analgesic effects in response to alcohol may involve learning mechanisms as observed with tolerance to other actions of alcohol.
2.2. Pain, chronic excessive drinking and alcohol dependence
Extended periods of alcohol exposure induce pain symptoms and exacerbate chronic pain arising from other sources. Alcoholism is typically accompanied by the emergence of negative emotional states that constitute a motivational withdrawal syndrome when access to alcohol is disrupted (Gilpin and Koob, 2008; Koob, 2003). Chronic alcohol use impacts several peripheral and central nervous system actions, and while it has long been observed that oral alcohol administration increases human pain thresholds, withdrawal from chronic use often increases pain sensitivity as one component of a larger alcohol withdrawal syndrome (Jochum et al., 2010). Prolonged and excessive alcohol exposure itself generates a small fiber peripheral neuropathy in both rodents and humans (Mellion et al., 2011). Indeed, the development of neuropathy during alcohol withdrawal may represent one critical, definitive symptom indicative of dependence clinically distinct from alcohol abuse (Diamond and Messing, 1994). In this regard, reduced nociceptive thresholds gradually emerge in ethanol-dependent rats relative to non-dependent controls (Dina et al., 2006; Edwards et al., 2012). These data suggest that drinking in alcoholics may be motivated in part by a desire to alleviate ethanol withdrawal-induced hyperalgesia. Whether alcohol alleviates neuropathic pain induced by chronic alcohol use remains to be demonstrated.
Self-reports of alcohol use specifically for pain management are common, even in younger adults (e.g., Riley and King, 2009). For example, Brennan et al. (2005) found that problem drinkers not only described more severe pain symptoms compared to nondrinkers, they also reported a higher incidence of using alcohol to manage their pain symptoms. Moreover, follow-up studies indicated that the use of alcohol to treat pain resulted in a worsening of drinking and other health-related problems three years later, suggesting that motivational mechanisms associated with painrelated drinking could underlie the persistence and exacerbation of alcohol-related disorders and overall lifetime morbidity. Rodent studies reveal elevated tail-flick thresholds (i.e., analgesia) in the hot plate test during chronic ethanol administration and hyperalgesia upon alcohol withdrawal that is subsequently reversed when alcohol is administered (Edwards et al., 2012; Gatch, 2009).
These examples support the plausibility that alcohol’s analgesic effects contribute to excessive alcohol use. Ongoing alcohol administration appears to alleviate some forms of pain, particularly during acute withdrawal, while generating a painful neuropathy that may not be alleviated by acute ethanol administration. Time course studies involving a wider variety of pain assays, especially those distinguishing sensory and affective aspects of pain, are needed to profile pain and analgesia resulting from alcohol drinking associated with dependence.
3. Chronic pain and neural substrates of alcohol addiction
The many brain regions contributing to pain reflect the pain’s adaptive function in transmitting actual or potentially harmful stimulation and the recruitment of cognitive, affective and motivated behavior necessary to respond to such stimuli. By implication, functional changes in pain pathways could also influence other functions supported by non-primary pain systems as indicated by higher incidences of depression and anxiety in people with chronic pain disorders (Fröhlich et al., 2006; Gureje, 2008). Analgesic responses to addictive drugs have been observed to covary with the drug’s reinforcing effects possibly because of similar neuroreceptor actions. Pain-related neural substrates may also influence motivationally relevant aversive states (including hyperalgesia) associated with protracted alcohol withdrawal and excessive drinking. We now discuss several likely neurochemical and neuroanatomical substrates affecting both pain transmission and alcohol dependence.
Le Magnen et al. (1980) and Franklin (1998) proposed that the positive hedonic state produced by addictive drugs is associated with an indifference to pain because neural substrates of analgesia and neural substrates of reinforcement overlap. Some evidence suggests that alcohol’s analgesic effects are mediated by neuroreceptor systems involved in alcohol reinforcement. For example, pharmacological studies partially support the involvement the muopioid receptor (MOR) in alcohol’s analgesic effects (Boada et al., 1981; Campbell et al., 2006, 2007; Pohorecky and Shah, 1987) in addition to its reinforcing effects (e.g., Cunningham et al., 1998; Froehlich et al., 1990; Walker and Koob, 2008). Gene knockout studies implicate G protein-coupled inwardly rectifying potassium 2 (GIRK2) channels as a major signal transduction mechanism for analgesic actions of many different drug classes including alcohol (Blednov et al., 2003; Ikeda et al., 2000; Mitrovic et al., 2003) and also suggest a role in alcohol reward (Hill et al., 2003). In the following section, we discuss pain transmission functions of neuroanatomical structures participating in alcohol reinforcement and addiction. Overlapping neural substrates of pain transmission and alcohol reinforcement raise the possibility that aberrant pain processing associated with chronic pain could alter the pharmacology and neurochemistry of alcohol to influence the transition to alcohol addiction.
Chronic pain represents a condition where nociception no longer acts as a useful, adaptive process beneficial to the organism, but instead represents a debilitating state commonly associated with negative emotional states and even affective disorders (Fröhlich et al., 2006; Gureje, 2008). Initial nociceptive sensitivity is often associated with hyperalgesic priming, a form of peripheral sensitization involving neuronal plasticity in primary afferent nociceptors (Reichling and Levine, 2009). In comparison, central sensitization mechanisms represent an augmented response commonly associated with pathological pain states and involve the propagated recruitment of central neurons in the nociceptive response, leading to a broadening of nociceptive field and amplification of pain processes (Woolf and Salter, 2000; Latremoliere and Woolf, 2009). Central sensitization of pain corresponds to the functional enhancement of nociceptive circuitry along the ascending neuraxis, including the dorsal horn of the spinal cord (Woolf, 1983), the rostroventral medulla (Porreca et al., 2002) and various limbic centers such as the central amygdala and prefrontal cortex (discussed below). In turn, functional gain in reinforcement-related limbic centers associated with a recruitment of stress (Koob and Le Moal, 2008) or immune system (Crews et al., 2011) factors may modify the central processing of nociceptive stimuli, resulting in aberrant plasticity linking pain and various affective disorders associated with the compulsive seeking of pain relief. Indeed, both pathological pain (Ji et al., 2003) and addiction (Nestler, 2001; Hyman, 2005) have been conceptualized as disorders of dysregulated neural plasticity involving mechanisms commonly ascribed to learning and memory processes. Consequently, functional enhancement of shared central circuitry following a history of excessive drinking or chronic pain may facilitate negative reinforcement, whereby compulsive drinking serves as a pain-reduction process. Thus, altered neural functioning associated with chronic pain conditions raises two issues: (1) whether chronic pain changes overlapping neural substrates of alcohol addiction and (2) whether these alterations are associated with increased alcohol drinking and the development of dependence.
3.1. Nucleus accumbens
Alcohol promotes DA release in the nucleus accumbens (NAc), a phenomenon contributing to the reinforcing effects of drugs of abuse including alcohol (Boileau et al., 2003; Di Chiara and Imperato, 1988). Opioid peptides in the NAc also have a key role in alcohol reward in animals and humans (Heyser et al., 1999; Mitchell et al., 2012). The NAc is currently understood to encode a full spectrum of responses—from reward to aversion—to a variety of motivationally salient stimuli. A series of studies in animals (Gear and Levine, 1995, 2011; Gear et al., 1999) provided evidence that noxious stimuli can actually induce heterosegmental antinociception through an ascending pathway originating from the spinal cord to the NAc. Here, subdermal capsaicin or paw immersion in hot water induced analgesia as measured by the jaw opening reflex. Such pain-induced antinociception is hypothesized to result from inhibition of tonic pronociceptive efferent activity from the NAc (possibly via the rostral ventral medulla) through dopaminergic and opioidergic receptor mechanisms (Gear and Levine, 1995, 2011; Gear et al., 1999). In humans, decreased BOLD signaling from baseline has been found in the NAc following the onset of painful stimulation (Aharon et al., 2006; Becerra et al., 2001; Becerra and Borsook, 2008). With respect to chronic pain, offset of noxious thermal stimulation elicited opposite patterns of NAc BOLD activity in chronic back pain patients compared with healthy control subjects (Baliki et al., 2010). These differences suggested that the reward value of pain relief associated with acute pain may also be mediated by the NAc. In addition, the functional connectivity of the NAc with other cortical regions was altered in chronic back pain patients. In healthy subjects, the NAc was mainly connected with the insula, whereas chronic back pain patients exhibited increased functional connectivity with the medial prefrontal cortex (mPFC). These studies suggest that the NAc mediates the reinforcing actions of alcohol as well as sensing the painful actions of noxious stimuli, possibly through overlapping neuropharmacological mechanisms. Functional changes in the NAc associated with chronic pain would, therefore, be predicted to affect alcohol reinforcement and addiction.
3.2. Central amygdala and prefrontal cortex
Converging evidence also suggests that neural substrates associated with motivationally relevant emotional aspects of alcohol withdrawal and dependence overlap with substrates of emotional aspects of nociceptive processing in areas such as the amygdala (Neugebauer et al., 2004) and prefrontal cortex (PFC; Vogt, 2005; Wei and Zhuo, 2001), where ascending pain pathways terminate for the processing of emotional components of pain. In particular, pain-responsive neurons are abundant in the lateral part of the central amygdala (known as the “nociceptive amygdala”; Bernard and Besson, 1990). In addition, this region is also critically important for alcohol reinforcement, and represents another possible neuroanatomical intersection of central pain modulation and alcohol reward. For example, intra-CeA administration of opioid receptor antagonists reduces oral alcohol self-administration in rats (Foster et al., 2004; Heyser et al., 1999). Alcohol also causes the release of several neurotransmitters in the CeA, including GABA, DA, and serotonin (Nie et al., 2000; Roberto et al., 2003; Yoshimoto et al., 2000). Thus, alcohol acts directly within this region of ascending nociceptive circuitry to possibly regulate neuronal plasticity related to the intersection of pain and negative affect.
Chronic pain produces multiple electrophysiological and molecular neuroadaptations in the CeA, a number of which are lateralized to the right CeA (e.g., Carrasquillo and Gereau, 2008; Ji and Neugebauer, 2009). The CeA receives functionally distinct inputs from the pontine parabrachial area (PB, nociceptive information) and basolateral amygdala (BLA, sensory-affective information) that are magnified in chronic pain states (Ikeda et al., 2007; Neugebauer et al., 2003). This plasticity is driven in part by an enhancement of glutamatergic systems, most notably activation of group I metabotropic glutamate receptors (mGluR1/mGluR5; Kolber et al., 2010; Li and Neugebauer, 2004; Neugebauer et al., 2003; Ren and Neugebauer, 2010). Neugebauer (2007) speculated that the amygdala facilitates nociceptive signaling in persistent pain states. Such altered processing may orient the organism’s motivational capacity to act toward alleviating this condition via heightened arousal (Koob et al., 1976) or negative reinforcement mechanisms (Koob and Le Moal, 2008). This function of pain contrasts with the stress-induced analgesia that is typically produced by acute stressors (Butler and Finn, 2009; also see Knoll and Carlezon, 2010). Relevant to the interface with alcoholism, chronic pain-induced activation of the amygdala is accompanied by alterations in mPFC function and production of cognitive deficits (Ji et al., 2010; Ji and Neugebauer, 2011; Sun and Neugebauer, 2011). Such executive system deficits are hypothesized to play a critical role in the aberrant decision-making that accompanies the transition from drug use to dependence (George and Koob, 2010), and by this mechanism individuals suffering from chronic pain may be more susceptible to alcohol misuse and poor pain management.
3.3. Insula
Functional neuroimaging studies in humans reveal the insular cortex to be the most consistently activated region during abnormal and induced pain experiences in humans (Tracey, 2011). These observations are consistent with studies in epilepsy patients in which direct electrical stimulation of distinct regions within the posterior insula evoked somatotopically specific pain sensations (Ostrowsky et al., 2002), and with neuroanatomical studies showing that the dorsal posterior insula receives pain input from a spinothalamocoritical circuit (Bernard et al., 1996). The anterior insula appears to integrate information about the salience of an impending painful stimulus. Aberrant misreading of such stimuli has been hypothesized to be a possible factor in the transition from acute to chronic pain (Wiech et al., 2010). The role of the insula in addiction was highlighted in a study showing that smokers with brain damage involving the insula were more likely to quit smoking without difficulty (Naqvi et al., 2007). A recent fMRI study showed that subjects with a family history of alcohol dependence and a variant of the GABRA2 gene associated with alcoholism had an elevated response in the insula when anticipating rewards and losses (Villafuerte et al., 2011). Whether the insula’s involvement in aspects of alcohol addiction is correlated with pain transmission remains unknown.
3.4. Chronic pain effects on drug reward
Although effects of chronic pain on the pharmacology and neurochemistry of alcohol self-administration have not been reported, several studies have shown that neuropathic pain alters the rewarding and reinforcing effects of opiates in rodent models. For example, spontaneous pain induced by nerve injury reduced morphine’s ability to induce conditioned place preferences (Ozaki et al., 2002, 2004) and suppressed the ability of morphine to lower brain stimulation reward (BSR) thresholds (Ewan and Martin, 2011). Similarly, dose–response curves for MOR agonists in maintaining self-administration were shifted to the right such that lower doses capable of maintaining heroin self-administration in normal rats were ineffective in a spinal nerve ligation model of spontaneous pain, whereas higher doses capable of reversing hypersensitivity to pain were self-administered (Martin et al., 2007). Because baseline BSR thresholds were unchanged by nerve injury, changes in heroin effects could not be attributed to general disruption of reward function. In light of alcohol’s effects on opioid systems, examining alcohol self-administration, particularly dose–response functions (see Carnicella et al., 2011) in chronic pain models such as these is warranted.
4. Neural dysregulation, alcohol dependence and chronic pain
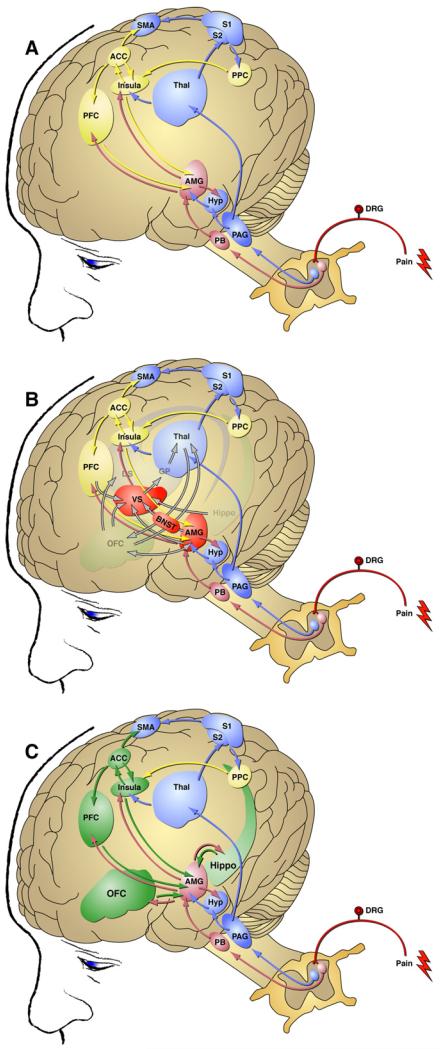
The transition from recreational alcohol use to alcohol dependence has been hypothesized to arise when brain reward and stress systems are dysregulated by chronic alcohol intoxication and withdrawal episodes (Koob and Le Moal, 2001; Edwards and Koob, 2010). This dysregulation engenders persistent negative emotional states and hypersensitivity to emotional distress when alcohol use is discontinued (Edwards and Koob, 2010). The transition to alcohol dependence involves a recruitment and/or potentiation of circuitry within forebrain areas including the amygdala and prefrontal cortex (Gilpin and Koob, 2008). Similarly, a sensitization of nociceptive circuitry (Reichling and Levine, 2009) in combination with a host of affective factors (Young Casey et al., 2008) is thought to drive the pathological transition from adaptive nociceptive signaling to chronic pain states. A common goal of both pain and addiction fields is to better understand transition-associated biochemical and behavioral neuroadaptations in hope of blocking or reversing the neuroplastic changes responsible for disease progression. It is becoming clear that neuroadaptively driven dysregulation of neural circuitry shared by pain and addiction processes may underlie a potential convergence of these two disease states. Fig. 1 illustrates the intersection of neural substrates mediating both nociception and alcohol dependence.
Fig. 1.
Intersection of neural substrates mediating nociception and alcohol dependence. (A) Ascending pathways mediating the supraspinal processing of pain. Blue structures are involved in the “fast” processing of pain via the spinothalamic tract and arrive indirectly at the amygdala. Pink structures are involved in the “fast” processing of pain via the spinoparabrachial-amygdala pathway that arrives directly at the amygdala. Yellow structures are involved in the “slower” cognitive/affective processing of pain. (B) Pathways for the supraspinal processing of pain superimposed on key elements of addiction circuitry implicated in negative emotional states. Addiction circuitry is composed of structures involved in the three stages of the addiction cycle: binge/intoxication (ventral striatum, dorsal striatum, and thalamus), withdrawal/negative affect (ventral striatum, bed nucleus of the stria terminalis, central nucleus of the amygdala; red structures), preoccupation/anticipation (prefrontal cortex, orbitofrontal cortex, and hippocampus). Note the significant neuroanatomical intersection of the supraspinal regulation of pain and addiction in the amygdala. (C) Regions involved in the preoccupation/anticipation stage of addiction (green) are accessed by ascending pain circuitry, and this interaction may promote compulsive alcohol seeking under conditions of acute or chronic pain. ACC, anterior cingulate cortex; AMG, amygdala; BNST, bed nucleus of the stria terminalis; DRG, dorsal root ganglion; DS, dorsal striatum; GP, globus pallidus; Hippo, hippocampus; Hyp, hypothalamus; Insula, insular cortex; OFC, orbitofrontal cortex; PAG, periaqueductal grey; PB, parabrachial nucleus; PFC, prefrontal cortex; PPC, posterior parietal cortex; S1, S2, somatosensory cortex; SMA, supplementary motor area; Thal, thalamus; VS, ventral striatum. Modified with permission from Blackburn-Munro and Blackburn-Munro (2003),Koob et al. (2008), and Shurman et al. (2010).
4.1. Neuroadaptation within the central amygdala
The CeA represents one potential node of intersection between nociception and motivationally relevant negative affect associated with alcohol dependence. Neuroadaptations in this region are particularly associated with the advanced progression of disease in animal models of pain and alcohol dependence. In terms of CeA excitability, alcohol increases glutamate levels in the CeA of alcohol-dependent, but not alcohol-naïve, animals (Roberto et al., 2004). Alcohol-dependent animals also display increased levels of the stress-related neuropeptide corticotropin-releasing factor (CRF) in the CeA during withdrawal (Merlo-Pich et al., 1995). Importantly, intra-CeA infusion of a CRF receptor antagonist reduces excessive levels of alcohol drinking in dependent animals without altering limited alcohol self-administration in non-dependent animals or water intake (Funk et al., 2006). Similar to animals with a history of alcohol dependence, animals having undergone arthritis induction display an enhanced CeA excitability that is reduced via CRF1 receptor antagonism (Ji and Neugebauer, 2007). Moreover, enhanced anxiety-like behavior following either alcohol withdrawal (Huang et al., 2010) or arthritis induction (Ji et al., 2007) is blocked following intra-CeA CRF1 receptor antagonism. Downstream of CRF1 receptors (Kageyama et al., 2007; Refojo et al., 2005), a recruitment of amygdala extracellular signal-regulated kinase (ERK) signaling has also been demonstrated during withdrawal in alcohol-dependent animals (Sanna et al., 2002) and is hypothesized also to be a critical component underlying pain-related synaptic plasticity and behavior (Carrasquillo and Gereau, 2007; Fu et al., 2008; Kolber et al., 2010). In contrast to CRF1 receptor signaling, activation of CeA CRF2 receptors may mediate the anti-nociceptive effects of CRF (Ji and Neugebauer, 2008) and this system may be responsible for the amygdala’s role in stress-induced analgesia described above. Consistent with this hypothesis, microinjection of a CRF2 receptor agonist into the CeA reduces excessive drinking in alcohol-dependent rats (Funk and Koob, 2007).
4.2. Cortical regulation of pain and addiction
Another central pain regulatory site, the PFC, is thought to play an important role in the maladaptive behaviors commonly observed in chronic drug users, including a high propensity for relapse (Lasseter et al., 2010). The PFC is an aggregate of several integrated regions, including the prelimbic, infralimbic, and anterior cingulate cortex (ACC). These regions receive information (including nociception-related information) primarily from the mediodorsal thalamus (Uylings and van Eden, 1990) and are collectively involved in driving drug-seeking behavior via their connections to other elements of drug- and drug context-induced relapse circuitries (Feltenstein and See, 2008; Janak and Chaudhri, 2010; Self and Nestler, 1998; Shaham et al., 2003). In regard to alcohol, alcohol-paired context-induced alcohol seeking is associated with enhanced Fos protein expression (a marker of neuronal activation) in the ACC of rats (Dayas et al., 2007), suggesting that potentiated cingulate activity could contribute to compulsive alcohol-seeking behaviors observed in dependent subjects. Similarly, the affective components of pain in humans are regulated by a pathway that includes the ACC (Price, 2000; Vogt, 2005). Particularly interesting is the proposed role of the ACC in organizing behavioral goals (such as avoiding negative emotional conditions) following nociceptive input (Vogt et al., 1997, 2004). Similar to the CeA, evidence for a specific role of ACC ERK signaling in the induction and expression of affective pain has been demonstrated in rodent models (Cao et al., 2009; Johansen et al., 2001; Wei and Zhuo, 2008).
4.3. Stress systems, pain and alcohol dependence
Alcohol dependence can be viewed as a condition whereby stress-related mechanisms come to predominate in driving compulsive alcohol seeking and excessive drinking (Breese et al., 2011; Koob et al., 2008; Uhart and Wand, 2009). Chronic stress can also induce a persistent hyperalgesic state in animal models (Imbe et al., 2006), indicating the intimate relationship between stress and pain and also suggesting a possible predisposing role of early life stress on aberrant nociception in adulthood (Davis et al., 2005; Green et al., 2011). Although mechanisms of nociception, stress, and anxiety are difficult to dissociate, pain represents a unique negative affective valence that is often intimately associated with a host of psychiatric disorders, including drug dependence (Elman et al., 2011). Some have even speculated that certain stress-related psychological disorders, including posttraumatic stress disorder and chronic fatigue syndrome, may feature generalized pain as a primary debilitating symptom (Reichling and Levine, 2009). Investigations have uncovered remarkable interactions between stress-induced potentiation of peripheral immune mediators and primary afferent nociceptor activation (Khasar et al., 2005). This stress-induced potentiation of nociception was mimicked by direct local glucocorticoid administration (Khasar et al., 2008). Elevated glucocorticoid levels are a hallmark of chronic alcohol consumption and represent one marker for relapse potential (Adinoff et al., 1998; Gianoulakis et al., 2003; Walter et al., 2006). Glucocorticoids also sensitize extrahypothalamic CRF systems in the CeA (Imaki et al., 1991; Makino et al., 1994; Swanson and Simmons, 1989). In support of a causal role for glucocorticoids, the glucocorticoid receptor antagonist mifepristone (RU38486) reversed both the chronic alcohol-induced hyperalgesia in binge-drinking rats (Dina et al., 2008) and the development of compulsive drinking in ethanol-dependent rats (Vendruscolo et al., 2012), suggesting a close association between excessive alcohol exposure, recruitment of stress signaling, and resultant dependence-like symptomatology, including pain. Supporting this hypothesis, blockade of the CRF1 receptor, a target commonly associated with stress- and anxiety-like behaviors, alleviates nociceptive hypersensitivity in a wide variety of animal pain assays (e.g., Hummel et al., 2010) including the hyperalgesia of alcohol withdrawal (Edwards et al., 2012) and also attenuates excessive limbic reactivity (including amygdala, ACC, and insula regions) during pain expectation in human irritable bowel syndrome patients (Hubbard et al., 2011).
5. Genetic influences on pain, alcohol analgesia and alcohol dependence
Pain sensitivity and alcohol analgesia are enhanced in alcohol dependent patients and FHP individuals and may also be altered in animal models of genetic vulnerability for alcohol dependence. In one study, alcohol administration enhanced tolerance for a painful electric shock only in FHN subjects (Perrino et al., 2008) whereas a more comprehensive study (Ralevski et al., 2010) found that FHP subjects scoring high for neuroticism displayed greater alcohol analgesia than FHN subjects and FHP subjects with low neuroticism scores. Studies in rodents selectively bred for differences in alcohol preference also provide partial evidence alcohol preference and pain response covary (Chester et al., 2002; Kampov-Polevoy et al., 1996; but see Kimpel et al., 2003). Given the possibility of a genetic link between pain processing and alcohol dependence, we suggest possible candidates having the potential to influence neurotransmitter systems involved in alcohol dependence and pain.
5.1. KCNJ6
A human study revealed an association between a polymorphism of the KCNJ6 gene, the gene encoding GIRK2, and alcohol dependence in an adult German sample (Clarke et al., 2011). Although pain responses and alcohol analgesia were not investigated in the study, a subsequent analysis in an adolescent population demonstrated that the same genotype was associated with hazardous drinking when early psychosocial stress was present. Given the possible link between psychosocial stress and pain disorders (e.g., Bardin et al., 2009; Grande et al., 2004; Nilsen et al., 2007; Scarinci et al., 1994; Van Houdenhove and Luyten, 2006), and the role of GIRK2 channels in the analgesic effects of alcohol and other drugs, exploring the relationship between the KCNJ6 polymorphism and pain transmission, including alcohol analgesia, in this population may further support a role for pain in the development of alcohol dependence.
5.2. OPRM1
The A118G variant of the μ-opioid receptor 1 (OPRM1) gene may also prove to influence pain sensitivity, alcohol reinforcement and analgesia although evidence for a relationship between the A118G variant and alcohol dependence is mixed (Bart et al., 2005; Franke et al., 2001; Kim et al., 2009; Nishizawa et al., 2006). A118G carriers do have diminished pain sensitivity (Fillingim et al., 2005; Lötsch et al., 2006, but see Vossen et al., 2010). The analgesic effects of alcohol have not been studied as a function of OPRM1 status, but a number of studies suggest carriers of the A118G variant or a functionally equivalent polymorphism show enhanced alcohol preference and euphoria (Barr et al., 2010; Ray and Hutchison, 2004) and enhanced striatal DA response to alcohol (Ramchandani et al., 2011), a response often associated with drug reward.
5.3. COMT
The val158met polymorphism of the catechol-O-methyl-transferase (COMT) gene results in higher COMT enzyme levels, lower D2 receptor-mediated neurotransmission and elevated activation of the MOR system. The met158 allele is associated with higher subjective and brain responses to pain (Mobascher et al., 2010) and suppressed MOR response to pain (Zubieta et al., 2003). Whether the COMT met158 allele influences alcohol dependence remains unclear. Some studies suggest that the met158 allele contributes to the development of both late-onset and early-onset alcoholism (Tiihonen et al., 1999; Wang et al., 2001) whereas others fail to find an association between COMT variation and alcohol dependence (Foroud et al., 2007; Ishiguro et al., 1999; Samochowiec et al., 2006). Male COMT −/− mice were found to drink more alcohol than wildtype mice (Tammimäki et al., 2008); however, it cannot be concluded yet whether this reflects stronger or weaker reinforcing effects.
6. Allostatic load, chronic emotional pain, and alcohol dependence
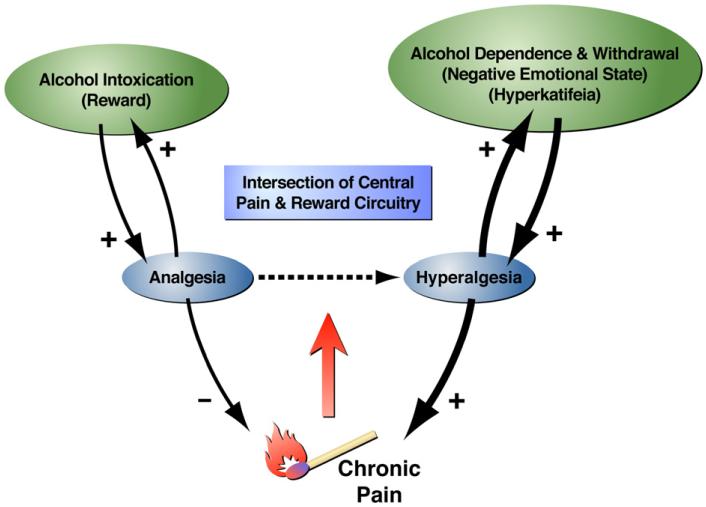
The brain and body respond to events such as alcohol intoxication, stress, and injury by activating neuronal and hormonal responses to promote physiological stability in the face of a changed set point (allostasis). When these events are frequent or severe, as with chronic excessive alcohol intoxication and withdrawal episodes, the stabilizing responses become dysregulated (allostatic load) as result of structural and functional changes in the brain to engender an enduring pathophysiological state (Koob and Le Moal, 2001; McEwen, 2000). Within this framework, we hypothesize that alcohol triggers an initial rewarding and analgesic response (acute intoxication) followed by an opposing dysphoric and hyperalgesic state (acute withdrawal). More specifically, extended episodes of alcohol intoxication and withdrawal alter brain stress circuitry (including CRF signaling within the CeA) and reward circuitry (including DA signaling in the VTA and NAc), and as a result, are hypothesized to engender a persistent negative affective state, including a hypersensitivity to emotional distress termed hyper-katifeia (Shurman et al., 2010) that motivates excessive alcohol consumption in an effort to restore the normal hedonic/emotional state (Koob and Le Moal, 2001; Koob, 2003). Protracted exposure to dependence-inducing alcohol concentrations followed by repeated withdrawals also heightens sensitivity to mechanical stimulation through CRF1-receptor dependent mechanisms (Edwards et al., 2012). This suggests that emotional pain (hyperkatifeia) and sensory pain (hyperalgesia) resulting from allostatic-like dysregulation of overlapping pain and addiction pathways could contribute to excessive alcohol use (Fig. 2). In this sense, it has been suggested that addiction could be considered a type of chronic emotional pain syndrome (Koob and Le Moal, 2006, p. 449).
Fig. 2.
Schematic diagram describing the hypothesized role of chronic pain in facilitating alcohol dependence. Although alcohol consumption produces analgesia, excessive use promotes hyperalgesia that may represent a negative and pathological emotional state associated with dependence. In turn, we propose that negative emotional states associated with drug withdrawal and protracted abstinence can also exacerbate dysregulated nociception. Hyperalgesia (increased sensitivity to pain) caused by protracted and excessive alcohol intake could therefore be closely associated with the parallel development of hyperkatifeia (increased sensitivity to negative emotions; Shurman et al., 2010).
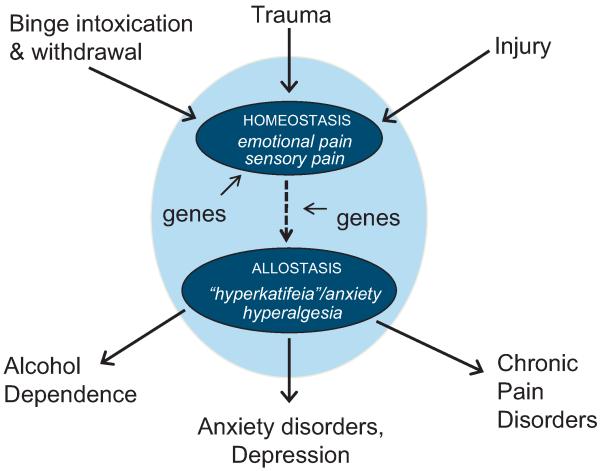
Allostatic-like dysregulation of shared neurocircuitries and neurochemicals has been invoked to explain vulnerability to alcohol addiction resulting from chronic alcohol and stress (Breese et al., 2011; Uhart and Wand, 2009), as well as comorbidity between depression and pain disorders (Robinson et al., 2009). We propose a model (Fig. 3) where alcohol, stressors and injury are similarly capable of dysregulating a common set of neural substrates (including the CeA, NAc, ACC, and insula; symbolized by the outer oval in Fig. 3) to engender a heightened state of sensitivity to emotional and sensory pain. Under normal, homeostatic conditions (symbolized by the upper inner oval), alcohol intoxication produces emotional and sensory pain though a compensatory opponent response (i.e., withdrawal) to an initial rewarding and analgesic action whereas emotional and sensory pain comprise the initial response to trauma and injury. Chronic alcohol intoxication and withdrawal, intense and/or untreated injury, or intense and/or unresolved trauma are each capable of increasing allostatic load (indicated by the dashed arrow) to the extent that a dysfunctional state emerges (symbolized by the lower inner oval) characterized by persistent hyperkatifeia and hyperalgesia. We hypothesize that the sensory and emotionally based allostatic state serves as a predisposing condition, as well as a shared phenotypic characteristic of alcohol dependence, anxiety and depression, and chronic pain disorders. We suggest that full expression of these distinct disease states may depend on between-systems interactions in which the shared neural circuitry illustrated in this model influences systems exclusive to a single disorder or subset of disorders. Shared neurocircuitry and neurochemistry enables crosstalk between the diverse disorders such that changes in neural structure and function (i.e., allostatic load) arising from one disorder can affect the others. The model accounts for well-documented comorbidities between alcohol and anxiety disorders (Kushner et al., 2012), anxiety, depression and chronic pain disorders (Gerrits et al., 2012; Gureje et al., 2008) as well as alcohol dependence and pain sensitivity discussed previously. It also predicts that drugs (such as CRF-1 receptor antagonists) acting upon the shared neurocircuits would likely be effective for treating alcohol dependence and pain disorders whereas other pharmacotherapies targeting disorder-specific mechanisms would be effective for one disorder, but not the others. The model also explains observed functional substitutability of acute alcohol withdrawal episodes and restraint stress in provoking social anxiety (Breese et al., 2005).
Fig. 3.
A model of how alcohol intoxication and withdrawal, trauma (stress) and injury transition to the corresponding disease states of alcohol dependence, anxiety disorders/depression, and chronic pain through actions upon an overlapping set of neural circuits (symbolized by the outer oval). Under normal, homeostatic conditions (symbolized by the upper inner oval), alcohol intoxication produces emotional and sensory pain though a compensatory opponent response (i.e., withdrawal) to an initial rewarding and analgesic action. Emotional and sensory pain comprise the initial response to trauma and injury that may be followed by a compensatory analgesic/euphoric response when terminated. When the initiating condition is not resolved (i.e., chronic alcohol intoxication and withdrawal, untreated injury) homeostasis can no longer be sustained. Neural circuits become dysregulated (indicated by the dashed arrow) resulting in an allostatic state (symbolized by the lower inner oval) characterized by persistent hyperkatifeia and hyperalgesia. According to the model, chronic alcohol intoxication and withdrawal increases allostatic load and results in hyperkatifeia and hyperalgesia. Vulnerability to develop chronic pain disorders following intense and/or untreated injury is increased because the ability to restore physiologic stability is compromised by dysregulated neural circuits. Similarly, the model predicts that intense and/or untreated injury increases allostatic load through similar neural mechanisms enhancing vulnerability to alcohol dependence by affecting relevant alcohol actions upon dysregulated neural circuits. As illustrated in the model, intense and unresolved trauma is also predicted to contribute to allostatic load in this system to influence vulnerability to chronic pain disorders and alcohol dependence. The model also acknowledges the role of genetic influences, such as those discussed previously, on the initial homeostatic responses, as well as the parameters involved in the development of allostatic load.
7. Further considerations
The proposed allostatic emotional state model is supported by observations that pain transmission and emotional aspects of alcohol dependence involve overlapping neurocircuitry and that alcohol dependent and FHP individuals are more sensitive to painful stimulation. It is also consistent with observed affective disorders associated with alcohol dependence and pain disorders, use of alcohol to cope with pain (Brennan et al., 2005; Sheu et al., 2008), affective pain experience as a significant predictor of alcohol use (Lawton and Simpson, 2009), and significant positive associations between alcohol dependence and chronic pain (Demyttenaere et al., 2007; Von Korff et al., 2005). In contrast to our model’s predictions, however, some investigators do not find greater-than-chance co-occurrence rates of pain and alcohol use disorders (Dersh et al., 2007) and may even find less frequent alcohol use (e.g., Thelin Bronner et al., 2012) despite more alcohol drinking problems (e.g.,Brennan et al., 2011). These findings are understandable if one considers that there are conditions in which excessive alcohol drinking or the presence of chronic pain does not induce an allostatic-like negative emotional state.
For example, a significant proportion of people meeting diagnostic criteria for alcohol dependence spontaneously reduce their drinking (Dawson et al., 2005). Therefore, extended periods of excessive alcohol drinking meeting diagnostic criteria for alcohol dependence may not always dysregulate neural circuits associated with pain and negative emotional states. In animal models, chronic exposure with brief, daily withdrawal episodes produces more rapid increases in alcohol self-administration relative to continuous alcohol exposure and, by inference, a more rapid escalation of the allostatic processes responsible for excessive ethanol self-administration (O’Dell et al., 2004). Binge-like drinking models with intermittent withdrawal episodes also produce a more rapid development of hyperalgesia vs. continuous access models (e.g.,Dina et al., 2006 vs. Dina et al., 2000). Repeated acute withdrawal episodes, therefore, may be necessary to activate neuroplasticity necessary for the induction of an allostatic-like negative emotional state, a conclusion that is consistent with greater incidence of prior delirium tremens episodes in patients experiencing pain problems relative to those not reporting pain (Trafton et al., 2004).
Although negative emotional states have been observed in laboratory models of chronic pain (Hummel et al., 2008; Narita et al., 2006; Parent et al., 2012), studies using a neuropathic pain model (e.g., Ewan and Martin, 2011) failed to produce increases in BSR thresholds, thought to reflect the hypohedonia following withdrawal from several drug classes including alcohol (Koob, 2003, 2009). Therefore, conditions necessary for painful stimulation to engage allostatic mechanisms relevant to hyperkatifeia and hyperalgesia require further study. In this context, a history of drug use or stress associated with unresolved pain may be key drivers of allostatic load.
Personality and social influences may influence the expression of allostatic-like negative emotional states and the subsequent development of alcohol dependence and pain disorders. For example, personality disorders linked to alcohol use disorders such as anxiety disorders (Grant et al., 2004b) and somatization disorders (Hasin and Katz, 2007), are also associated with increased pain sensitivity and pain pathology (Dersh et al., 2001) whereas other personality disorders associated with alcohol dependence such as borderline personality disorder, and antisocial personality disorder are characterized by lower sensitivity to pain (Schmahl et al., 2006). These contrasting relationships may reflect divergent behavioral and physiological responses that have evolved to accommodate psychosocial challenges and their corresponding influences on allostatic load and vulnerability to stress-related disease (Korte et al., 2005). Divergent genetic, physiological, and behavioral stress responses are also likely to differentially interact with alcohol intoxication and withdrawal as well as exposure to painful stimulation. In addition to biological mediators, the influence of contextual risks and resources (e.g., SES, social support) and cognition (e.g., appraisal and coping) has been incorporated into recent allostasis models (Ganzel et al., 2010). These variables may exert their influence by modulating stress-induced neuroplasticity in the amygdala, hippocampus and prefrontal cortex, structures implicated in pain and alcohol dependence (Davidson and McEwen, 2012). It is important, therefore, that research on the alcohol–pain relationship consider the moderating influences of personality, social context and cognitive style. In this light, interpersonal resources were found to moderate the effects of painful medical conditions on drinking frequency (Brennan et al., 2011).
As discussed in Ilgen et al. (2010), the co-occurrence of pain and alcohol dependence may be explained by a number of hypotheses including: (a) alcohol use to alleviate sensory aspects of pain (self-medication), (b) activation of underlying predispositions for alcohol dependence by stress created by chronic pain (stress-diathesis), (c) underlying risk factors common to both pain and alcohol use disorders, and (d) development of pain conditions as the result of injuries incurred while intoxicated. Temporal onsets of pain and alcohol use disorders consistent with each of these hypotheses have been reported in the literature making it unlikely that a single comorbidity hypothesis applies to all individuals. For example, consistent with self-medication and stress-diathesis accounts, some find that chronic pain increases subsequent risk for alcohol drinking problems or the onset of a new substance use disorder (Brown et al., 1996; Larson et al., 2007). Others report that alcohol and substance use disorders precede the onset of chronic pain conditions (e.g., Dersh et al., 2007), a sequence that is consistent with the typical age of onset of the two conditions in the general population.
By stipulating that the allostatic state arising through actions by alcohol, trauma (stress) or injury does not depend on the temporal sequence of exposure (i.e., the insults are functionally substitutable) our model is compatible with many hypotheses. Nevertheless, laboratory studies suggest that the presence of hyperkatifeia and enhanced responsiveness to painful stimulation may not always be sufficient to increase alcohol drinking. For example, early animal studies on the relationship between alcohol dependence and withdrawal and subsequent self-administration generally yielded equivocal findings most likely because reinforcing effects of alcohol were not established prior to dependence induction (see Heilig et al., 2010; Roberts et al., 2000). Alcohol use (quantity and frequency) and withdrawal history is predicted to be an important determinant of whether allostatic-like negative emotional states induced by chronic pain or stress affect drinking and contribute to the development and maintenance of alcohol dependence.
The model presented here raises important considerations for future studies of the pain–alcohol relationship in clinical populations. First, we suggest that valid measures of relevant allostatic-like negative emotional states such as exaggerated brain responses to negative affective stimuli (Gilman and Hommer, 2008) be developed and implemented. Although positive associations between chronic pain and substance use disorders have been reported in the absence of mood and anxiety disorders (Ilgen et al., 2010)—a finding seemingly at odds with our hypothesis—we propose that hyperkatifeia, rather than full blown affective disorder, is the common antecedent and phenotype associated with pain disorders and alcohol dependence. In addition, comprehensive measures of past alcohol use patterns, including quantity and frequency measures and measures of withdrawal experiences, will enhance interpretation of temporal onset studies.
Finally, an enormous wealth of evidence has pointed to a substantial overlap in the biobehavioral mechanisms underlying alcohol and opioid dependence that may closely relate to a common dysregulation of pain signaling. Opioids and alcohol activate similar neuronal circuitry (Koob and Bloom, 1988; Herz, 1997; Siggins et al., 2003; Modesto-Lowe and Fritz, 2005; Gianoulakis, 2009), and also share a close similarity with regard to withdrawal symptomatology (West and Gossop, 1994) including mechanical hypersensitivity in animal models of alcohol and opioid dependence (e.g., Edwards et al., 2012). Indeed, mice that display an alcohol deprivation effect (increased drinking levels as a consequence of withdrawal) also display greater hyperalgesia during concomitant morphine withdrawal (Salimov et al., 1993). For the past several decades, clinicians have been required to balance the use of highly effective pain-relieving opioid medications with their potential for generating a paradoxical hyperalgesia (Simonnet and Rivat, 2003; Angst and Clark, 2006) and a transition to dependence in susceptible individuals (Fishbain et al., 2008; Turk et al., 2008). The rise in prescription opioid dependence (Maxwell, 2011) further suggests a link between pain modulation and addiction. Ren et al. (2009) found that a hyperalgesic state can persist for up to five months in abstinent opioid addicts, while addicts with more pain sensitivity also displayed greater cue-induced drug craving at this time point. Given that chronic pain is well known to cause both emotional distress and produce a sustained negative emotional state (King et al., 2009), both alcohol- and opioid-induced hyperalgesia may constitute conditions intimately associated with the transition to drug dependence by facilitating negative reinforcement processes. Consequently, abstinent individuals with a history of opioid or alcohol dependence may be susceptible to cross-sensitization mechanisms driving relapse to continued use of either drug. In contrast to animals made dependent on heroin or ethanol, a development of mechanical hyperalgesia was not observed in rats that displayed escalated cocaine intake (Edwards et al., 2012). Furthermore, a recent study found that induction of a chronic pain-like state induced by spinal nerve ligation was able to block morphine-induced, but not cocaine-induced, potentiation of rewarding electrical brain stimulation (Ewan and Martin, 2011), suggesting a lack of association between exaggerated nociception and cocaine reward. Investigations into the differential ability of abused substances to interact with nociceptive signaling will enhance our knowledge of the precise mechanisms underlying the intersection of pain signaling and the potential transition to addicted states.
8. Remaining questions
Our model raises additional questions centered on three fundamental issues: (1) What is the spectrum of sensory and affective pain responses associated with alcohol dependence, stress-related affective disorders, and chronic pain disorders? (2) What functional neuroanatomical and neurochemical changes of circuitry are mutually affected by alcohol intoxication and withdrawal, trauma and other stressors, and injury and other insults leading to hyperalgesia and hyperkatifeia; (3) What are the structural and functional mechanisms of allostatic dysregulation in these circuits associated with alcohol exposure and withdrawal, stress, and pain-inducing injuries. Regarding the first issue, in addition to functional neuroimaging approaches in humans (Tracey, 2011), animal models continue to be developed to measure a broad range of pain responses (Mogil, 2009). Important to our hypothesis, models purporting to measure cognitive and affective aspects of pain would be useful including paradigms measuring effects of fear- or anxiety-inducing stimuli on reflexive pain measures as well as conditioned responses to painful stimuli. The second issue involves whether neuroanatomical substrates mutually affecting alcohol dependence and pain are shared regions of interest or truly represent overlapping neural circuits. Studies using neuroimaging techniques to identify patterns of neural activation associated with chronic pain conditions and alcohol dependence are needed in addition to animal studies measuring the effects of experimental manipulation of brain pathways on pain and alcohol response measures in the same subjects. Finally, we suggested that alcohol intoxication and withdrawal, stress, and some forms of neuronal injury may be functionally substitutable in terms of their effects on pain and alcohol related measures, possibly through distinct mechanisms, because they accelerate allostatic load in systems relevant to emotional and affective pain. An important implication of this hypothesis is that identifying neuropathology engendering elevated sensitivity to sensory and affective pain and the full spectrum of interactions with disease specific mechanisms may be a critical step in the prevention and treatment of alcohol dependence and diverse, comorbid conditions arising from aberrant pain transmission.
Acknowledgements
Preparation of this review was partially supported by funds from the National Institute on Alcohol Abuse and Alcoholism (AA008459, AA012602, AA006420, AA007456, and AA020608, GFK), a National Research Service Award (AA018250, SE), and by the Pearson Center for Alcoholism and Addiction Research at The Scripps Research Institute. We also thank Janet Hightower and Mike Arends for graphic and editorial assistance. This is article number 21689 from The Scripps Research Institute.
Footnotes
The authors declare no conflicts of interest.
References
- Adinoff B, Iranmanesh A, Veldhuis JD, Fisher L. Disturbances of the stressresponse: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health and Research World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Aharon I, Becerra L, Chabris CF, Borsook D. Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neuroscience Letters. 2006;392:159–164. doi: 10.1016/j.neulet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin L, Malfetes N, Newman-Tancredi A, Depoortère R. Chronic restraint stress induces mechanical and cold allodynia, and enhances inflammatory pain in rat: relevance to human stress-associated painful pathologies. Behavioural Brain Research. 2009;205:360–366. doi: 10.1016/j.bbr.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Barr CS, Chen SA, Schwandt ML, Lindell SG, Sun H, Suomi SJ, Heilig M. Suppression of alcohol preference by naltrexone in the rhesus macaque: a critical role of genetic variation at the micro-opioid receptor gene locus. Biological Psychiatry. 2010;67:78–80. doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene 118 polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. European Journal of Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. Journal of Neurophysiology. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio-amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Progress in Brain Research. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends in Endocrinology and Metabolism. 2003;14:20–27. doi: 10.1016/s1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada J, Feria M, Sanz E. Inhibitory effect of naloxone on the ethanol-induced antinociception in mice. Pharmacological Research Communications. 1981;13:673–678. doi: 10.1016/s0031-6989(81)80055-0. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology and Therapeutics. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, Moos RH. Painful medical conditions and alcohol use: a prospective study among older adults. Pain Medicine. 2011;12:1049–1059. doi: 10.1111/j.1526-4637.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Cutter HSG. Alcohol, customary drinking behavior and pain. Journal of Abnormal Psychology. 1977;86:179–188. doi: 10.1037/0021-843X.86.2.179. [DOI] [PubMed] [Google Scholar]
- Brown RL, Patterson JJ, Rounds LA, Papasouliotis O. Substance abuse among patients with chronic back pain. Journal of Family Practice. 1996;43:152–160. [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Progress in Neurobiology. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Research. 2006;1097:71–77. doi: 10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcoholism, Clinical and Experimental Research. 2007;31:1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Cao H, Gao YJ, Ren WH, Li TT, Duan KZ, Cui YH, Cao XH, Zhao ZQ, Ji RR, Zhang YQ. Activation of extracellular signal-regulated kinase in the anterior cingulated cortex contributes to the induction and expression of affective pain. Journal of Neuroscience. 2009;29:3307–3321. doi: 10.1523/JNEUROSCI.4300-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Yowell QV, Ron D. Regulation of operant oral ethanol self-administration: a dose–response curve study in rats. Alcoholism, Clinical and Experimental Research. 2011;35:116–125. doi: 10.1111/j.1530-0277.2010.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RWT. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. Journal of Neuroscience. 2007;27:1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo RC, MacKenzie EA, Wegner ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Chester JA, Price CS, Froehlich JC. Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcoholism, Clinical and Experimental Research. 2002;26:19–27. [PubMed] [Google Scholar]
- Clarke TK, Laucht M, Ridinger M, Wodarz N, Rietschel M, Maier W, Lathrop M, Lourdusamy A, Zimmermann US, Desrivieres S, Schumann G. KCNJ6 is associated with adult alcohol dependence and involved in gene × early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology. 2011;36:1142–1148. doi: 10.1038/npp.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Advancing Pain Research, Care, and Education, Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin LY. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity. 2011;25:S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology. 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Cutter HSG, Malouf B, Kurtz NR, Jones WC. Feeling no pain. Journal of Studies on Alcohol. 1976;37:273–277. doi: 10.15288/jsa.1976.37.273. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clinical Journal on Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129:332–342. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Dersh J, Gatchel RJ, Polatin P. Chronic spinal disorders and psychopathology: research findings and theoretical considerations. Spine Journal. 2001;1:88–94. doi: 10.1016/s1529-9430(01)00017-1. [DOI] [PubMed] [Google Scholar]
- Dersh J, Mayer T, Theodore BR, Polatin P, Gatchel RJ. Do psychiatric disorders first appear preinjury or postinjury in chronic disabling occupational spinal disorders? Spine. 2007;32:1045–1051. doi: 10.1097/01.brs.0000261027.28779.52. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Messing RO. Neurologic effects of alcoholism. Western Journal of Medicine. 1994;161:279–287. [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. Journal of Neuroscience. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. European Journal of Neuroscience. 2008;27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. European Journal of Neuroscience. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurology. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Archives of General Psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology. 2011;114:624–632. doi: 10.1097/ALN.0b013e31820a4edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. British Journal of Pharmacology. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the muopioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. Journal of Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drugrelated behaviors? A structured evidence-based review. Pain Medicine. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Foroud T, Wetherill LF, Dick DM, Hesselbrock V, Nurnberger JI, Jr., Kramer J, Tischfield J, Schuckit M, Bierut LJ, Xuei X, Edenberg HJ. Lack of association of alcohol dependence and habitual smoking with catechol-O-methyltransferase. Alcoholism, Clinical and Experimental Research. 2007;31:1773–1779. doi: 10.1111/j.1530-0277.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, June HL. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Franke P, Wang T, Nothen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W. Nonreplication of association between mu-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. American Journal of Medical Genetics. 2001;105:114–119. [PubMed] [Google Scholar]
- Franklin KBJ. Analgesia and abuse potential: an accidental association or a common substrate? Pharmacology Biochemistry and Behavior. 1998;59:993–1002. doi: 10.1016/s0091-3057(97)00535-2. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li T-K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacology Biochemistry and Behavior. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Fröhlich C, Jacobi F, Wittchen HU. DSM-IV pain disorder in the general population. An exploration of the structure and threshold of medically unexplained pain symptoms. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:187–196. doi: 10.1007/s00406-005-0625-3. [DOI] [PubMed] [Google Scholar]
- Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Molecular Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Research. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. Journal of Neuroscience. 2006;44:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: integrating models of stress from the social and life sciences. Psychological Review. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Lal H. Effects of ethanol administration and withdrawal on thermal nociception in rats. Alcoholism, Clinical and Experimental Research. 1999;23:328–333. [PubMed] [Google Scholar]
- Gatch MB. Ethanol withdrawal and hyperalgesia. Current Drug Abuse Reviews. 2009;2:41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. Journal of Neuroscience. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear RW, Levine JD. Antinociception produced by an ascending spinosupraspinal pathway. Journal of Neuroscience. 1995;15:3154–3161. doi: 10.1523/JNEUROSCI.15-04-03154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Experimental Neurobiology. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;153:429–436. doi: 10.1016/j.pain.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Current Topics in Medicinal Chemistry. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic–pituitary–adrenal axis and pituitary b-endorphin as a function of alcohol intake, age and gender. Alcoholism, Clinical and Experimental Research. 2003;27:410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addiction Biology. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Overview: neurobiology of alcohol dependence with a focus on motivational mechanisms. Alcohol Research and Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disability and Rehabilitation. 1999;2:23–30. doi: 10.1080/096382899298061. [DOI] [PubMed] [Google Scholar]
- Grande LA, Loeser JD, Ozuna J, Ashleigh A, Samii A. Complex regional pain syndrome as a stress response. Pain. 2004;110:495–498. doi: 10.1016/j.pain.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004a;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004b;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O. Comorbidity of pain and anxiety disorders. Current Psychiatry Reports. 2008;10:318–322. doi: 10.1007/s11920-008-0051-0. [DOI] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Guimaraes Borges GL, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hasin D, Katz H. Somatoform and substance use disorders. Psychosomatic Medicine. 2007;69:870–875. doi: 10.1097/PSY.0b013e31815b00d7. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcoholism, Clinical and Experimental Research. 1999;23:1468–1476. [PubMed] [Google Scholar]
- Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology. 2003;169:108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Williamson O, Hogg M, Arnold C, Prosser A, Clements J, Konstantatos A, O’Donnell M. Predictors of pain severity 3 months after serious injury. Pain Medicine. 2010;11:990–1000. doi: 10.1111/j.1526-4637.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress induced sensitization. Journal of Pharmacology and Experimental Therapeutics. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. Journal of Neuroscience. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Cummons T, Lu P, Mark L, Harrison JE, Kennedy JD, Whiteside GT. Pain is a salient stressor that is mediated by corticotropin-releasing factor-1 receptors. Neuropharmacology. 2010;59:160–166. doi: 10.1016/j.neuropharm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons TA, Whiteside GT. The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain. 2008;140:436–445. doi: 10.1016/j.pain.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. American Journal of Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi T, Kumanishi T, Niki H, Yano R. Involvement of G-protein-activated inwardly rectifying K+ (GIRK) channels in opioid induced analgesia. Neuroscience Research. 2000;38:113–116. doi: 10.1016/s0168-0102(00)00144-9. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton J. The timing of onset of pain and substance use disorders. American Journal on Addictions. 2010;19:409–415. doi: 10.1111/j.1521-0391.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. Journal of Neuroscience. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Frontiers in Bioscience. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Haruo Shibuya T, Toru M, Saito T, Arinami T. Association study between high and low activity polymorphism of catechol-O-methyltransferase gene and alcoholism. Psychiatric Genetics. 1999;9:135–138. doi: 10.1097/00041444-199909000-00004. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N. The potent effect of environmental context on relapse to alcohol-seeking after extinction. Open Addiction Journal. 2010;3:76–87. doi: 10.2174/1874941001003010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. Journal of Neurophysiology. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Molecular Pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. Journal of Neurophysiology. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. Journal of Neurophysiology. 2009;102:2253–2264. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. Journal of Neurophysiology. 2011;106:2642–2652. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. Journal of Neuroscience. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in Neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bär KJ. Increased pain sensitivity in alcohol withdrawal syndrome. European Journal of Pain. 2010;14:713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen HA, Hole K. Learned tolerance to ethanol in the spinal cord. Pharmacology Biochemistry and Behavior. 1984;20:789–792. doi: 10.1016/0091-3057(84)90200-4. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Iwasaki Y, Sakihara S, Nigawara T, Kasckow J, Suda T. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. Journal of Endocrinology. 2007;195:199–211. doi: 10.1677/JOE-07-0125. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Overstreet DH, Rezvani AH, Viglinskaya IV, Badistov BA, Seredenin SB, Halikas JA, Sinclair JD. Pain sensitivity and saccharin intake in alcohol-preferring and -nonpreferring ratstrains. Physiology and Behavior. 1996;59:683–688. doi: 10.1016/0031-9384(95)02110-8. [DOI] [PubMed] [Google Scholar]
- Katon W, Egan K, Miller D. Chronic pain: lifetime psychiatric diagnoses and family history. American Journal of Psychiatry. 1985;142:1156–1160. doi: 10.1176/ajp.142.10.1156. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. Journal of Neuroscience. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, Oslin DW. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology. 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Kimpel MW, Brown MM, Froehlich JC. Pain thresholds in alcohol preferring and non-preferring rats: diurnal and repeated trial line differences. Alcoholism, Clinical and Experimental Research. 2003;27:1921–1928. doi: 10.1097/01.ALC.0000102720.08798.51. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature Neuroscience. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr. Dynorphin, stress, and depression. Brain Research. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, Gereau RW. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. Journal of Neuroscience. 2010;30:8203–8213. doi: 10.1523/JNEUROSCI.1216-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism, Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl. 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Fray PJ, Iversen SD. Tail-pinch stimulation: sufficient motivation for learning. Science. 1976;194:637–639. doi: 10.1126/science.982032. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; London; 2006. [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Everitt BJ, Robbins TW. Reward, motivation, and addiction. In: Squire LG, Berg D, Bloom FE, editors. Fundamental Neuroscience. 3rd ed. Academic Press; Amsterdam: 2008. pp. 987–1016. [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Wall MM, Krueger RF, Sher KJ, Maurer E, Thuras P, Lee S. Alcohol dependence is related to overall internalizing psychopathology load rather than to particular internalizing disorders: evidence from a national sample. Alcoholism, Clinical and Experimental Research. 2012;36:325–331. doi: 10.1111/j.1530-0277.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Current Topics in Behavioral Neurosciences. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. Journal of Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton J, Simpson J. Predictors of alcohol use among people experiencing chronic pain. Psychology, Health and Medicine. 2009;14:487–501. doi: 10.1080/13548500902923177. [DOI] [PubMed] [Google Scholar]
- Le Magnen J, Marfaing-Jallat P, Miceli D, Devos M. Pain modulating and reward systems: a single brain mechanism? Pharmacology Biochemistry and Behavior. 1980;12:729–733. doi: 10.1016/0091-3057(80)90157-4. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. Journal of Neurophysiology. 2004;91:13–24. doi: 10.1152/jn.00485.2003. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Stuck B, Hummel T. The human mu-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. Behavioral Neuroscience. 2006;120:1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Research. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug and Alcohol Review. 2011;30:264–270. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Mellion M, Gilchrist JM, De La Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle and Nerve. 2011;43:309–316. doi: 10.1002/mus.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropinreleasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobascher A, Brinkmeyer J, Thiele H, Toliat MR, Steffens M, Warbrick T, Musso F, Wittsack HJ, Saleh A, Schnitzler A, Winterer G. The val158met polymorphism of human catechol-O-methyltransferase (COMT) affects anterior cingulate cortex activation in response to painful laser stimulation. Molecular Pain. 2010;6:32. doi: 10.1186/1744-8069-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto-Lowe V, Fritz EM. The opioidergic-alcohol link: implications for treatment. CNS Drugs. 2005;19:693–707. doi: 10.2165/00023210-200519080-00005. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nature Reviews Neuroscience. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119L–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscie. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. The amygdala: different pains, different mechanisms. Pain. 2007;127:1–2. doi: 10.1016/j.pain.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RWT. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. Journal of Neuroscience. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. Journal of Pharmacology and Experimental Therapeutics. 2000;293:654–661. [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Westgaard RH, Stovner LJ, White LR, Bang Leistad R, Helde G, Rø M. Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients. European Journal of Pain. 2007;11:743–755. doi: 10.1016/j.ejpain.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Nishizawa D, Han W, Hasegawa J, Ishida T, Numata Y, Sato T, Kawai A, Ikeda K. Association of mu-opioid receptor gene polymorphism A118G with alcohol dependence in a Japanese population. Neuropsychobiology. 2006;53:137–141. doi: 10.1159/000093099. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism, Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguière F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cerebral Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in μ-opioid receptor functions in the ventral tegmental area. Journal of Neurochemistry. 2002;82:1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. Journal of Neurochemistry. 2004;88:1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Bérubé P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behavioural Brain Research. 2012;229:160–167. doi: 10.1016/j.bbr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino AC, Jr., Ralevski E, Acampora G, Edgecombe J, Limoncelli D, Petrakis IL. Ethanol and pain sensitivity: effects in healthy subjects using an acute pain paradigm. Alcoholism, Clinical and Experimental Research. 2008;32:952–958. doi: 10.1111/j.1530-0277.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky L, Shah P. Ethanol-induced analgesia. Life Sciences. 1987;41:1289–1295. doi: 10.1016/0024-3205(87)90208-6. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perrino A, Acampora G, Koretski J, Limoncelli D, Petrakis I. Analgesic effects of ethanol are influenced by family history of alcoholism and neuroticism. Alcoholism, Clinical and Experimental Research. 2010;34:1433–1441. doi: 10.1111/j.1530-0277.2010.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism, Clinical and Experimental Research. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Refojo D, Echenique C, Müller MB, Reul JM, Deussing JM, Wurst W, Sillaber I, Paez-Pereda M, Holsboer F, Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends in Neurosciences. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Neugebauer V. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Molecular Pain. 2010;16(6):93. doi: 10.1186/1744-8069-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, III, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. Journal of Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. Journal of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Fronteers in Bioscience. 2009;14:5031–5051. doi: 10.2741/3585. [DOI] [PubMed] [Google Scholar]
- Salimov R, Salimova N, Klodt P, Maisky A. Interaction between alcohol deprivation and morphine withdrawal in mice. Drug and Alcohol Dependence. 1993;34:59–66. doi: 10.1016/0376-8716(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jabłoński M, Rommelspacher H, Samochowiec A, Sznabowicz M, Horodnicki J, Sagan L, Pełka-Wysiecka J. Family-based and case–control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neuroscience Letters. 2006;410:1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Research. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Scarinci IC, McDonald-Haile J, Bradley LA, Richter JE. Altered pain perception and psychosocial features among women with gastrointestinal disorders and history of abuse: a preliminary model. American Journal of Medicine. 1994;97:108–118. doi: 10.1016/0002-9343(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Bohus M, Esposito F, Treede RD, Di Salle F, Greffrath W, Ludaescher P, Jochims A, Lieb K, Scheffler K, Hennig J, Seifritz E. Neural correlates of antinociception in borderline personality disorder. Archives of General Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Medicine. 2008;9:911–917. doi: 10.1111/j.1526-4637.2008.00420.x. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug seeking: neural and molecular mechanisms. Drug and Alcohol Dependence. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Medicine. 2010;11:1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Annals of the New York Academy of Sciences. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- Stewart S, Finn PR, Pihl RO. A dose–response study of the effects of alcohol on the perception of pain and discomfort due to electric shock in men at high familial-genetic risk for alcoholism. Psychopharmacology. 1995;119:261–267. doi: 10.1007/BF02246289. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. Journal of Comparative Neurology. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. Journal of Neurophysiology. 2011;106:960–973. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammimäki A, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Increase in free choice oral ethanol self-administration in catechol-o-methyltransferase gene-disrupted male mice. Basic and Clinical Pharmacology and Toxicology. 2008;103:297–304. doi: 10.1111/j.1742-7843.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- Thelin Bronner KB, Wennberg P, Källmén H, Schult ML. Alcohol habits in patients with long-term musculoskeletal pain: comparison with a matched control group from the general population. International Journal of Rehabilitation Research. 2012;35:130–137. doi: 10.1097/MRR.0b013e3283527d0d. [DOI] [PubMed] [Google Scholar]
- Tiffany S, McCal KJ, Maude-Griffin PM. The contribution of classical conditioning to tolerance to the antinociceptive effects of ethanol. Psychopharmacology. 1987;92:524–528. doi: 10.1007/BF00176489. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, Salonen JT, Ryynänen OP, Koulu M, Karvonen MK, Pohjalainen T, Syvälahti E, Hietala J. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Molecular Psychiatry. 1999;4:286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nature Reviews Neuroscience. 2011;7:173–181. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug and Alcohol Dependence. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clinical Journal of Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progress in Brain Research. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Van Houdenhove B, Luyten P. Stress, depression and fibromyalgia. Acta Neurologica Belgica. 2006;106:149–156. [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Wendy, Yau WY, Majczenko K, Zubieta JK, Zucker RA, Burmeister M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Molecular Psychiatry April. 2011 doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR. In: Handbook of Chemical Neuroanatomy. Bloom FE, Bjorkun A, Hokfelt T, editors. Elsevier; San Diego: 1997. pp. 455–528. [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. In: The Human Nervous System. 2nd ed. Paxinos G, Mai JK, editors. Academic Press; San Diego, CA: 2004. pp. 915–949. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical–mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Vossen H, Kenis G, Rutten B, van Os J, Hermens H, Lousberg R. The genetic influence on the cortical processing of experimental pain and the moderating effect of pain status. PLoS ONE. 2010;5:e13641. doi: 10.1371/journal.pone.0013641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Gerlach M, Weijers HG, Boening J, Wiesbeck GA. Cortisol concentrations, stress-coping styles after withdrawal, and long-term abstinence in alcohol dependence. Addiction Biology. 2006;11:157–162. doi: 10.1111/j.1369-1600.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Franke P, Neidt H, Cichon S, Knapp M, Lichtermann D, Maier W, Propping P, Nothen MM. Association study of the low-activity allele of catechol-O-methyltransferase and alcoholism using a family-based approach. Molecular Psychiatry. 2001;6:109–111. doi: 10.1038/sj.mp.4000803. [DOI] [PubMed] [Google Scholar]
- Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. Journal of Physiology. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhuo M. Activation of Erk in the anterior cingulated cortex during the induction and expression of chronic pain. Nature Reviews Neuroscience. 2008;4:28. doi: 10.1186/1744-8069-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DE, Haddox JD. Opioid pseudoaddiction – an iatrogenic syndrome. Pain. 1989;36:363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- West R, Gossop M. Overview: a comparison of withdrawal symptoms from different drug classes. Addiction. 1994;89:1483–1489. doi: 10.1111/j.1360-0443.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. Journal of Neuroscience. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuroscience – neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Ueda S, Kato B, Takeuchi Y, Kawai Y, Noritake K, Yasuhara M. Alcohol enhances characteristic releases of dopamine and serotonin in the central nucleus of the amygdala. Neurochemistry International. 2000;37:369–376. doi: 10.1016/s0197-0186(00)00037-1. [DOI] [PubMed] [Google Scholar]
- Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. 2008;134:69–79. doi: 10.1016/j.pain.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]