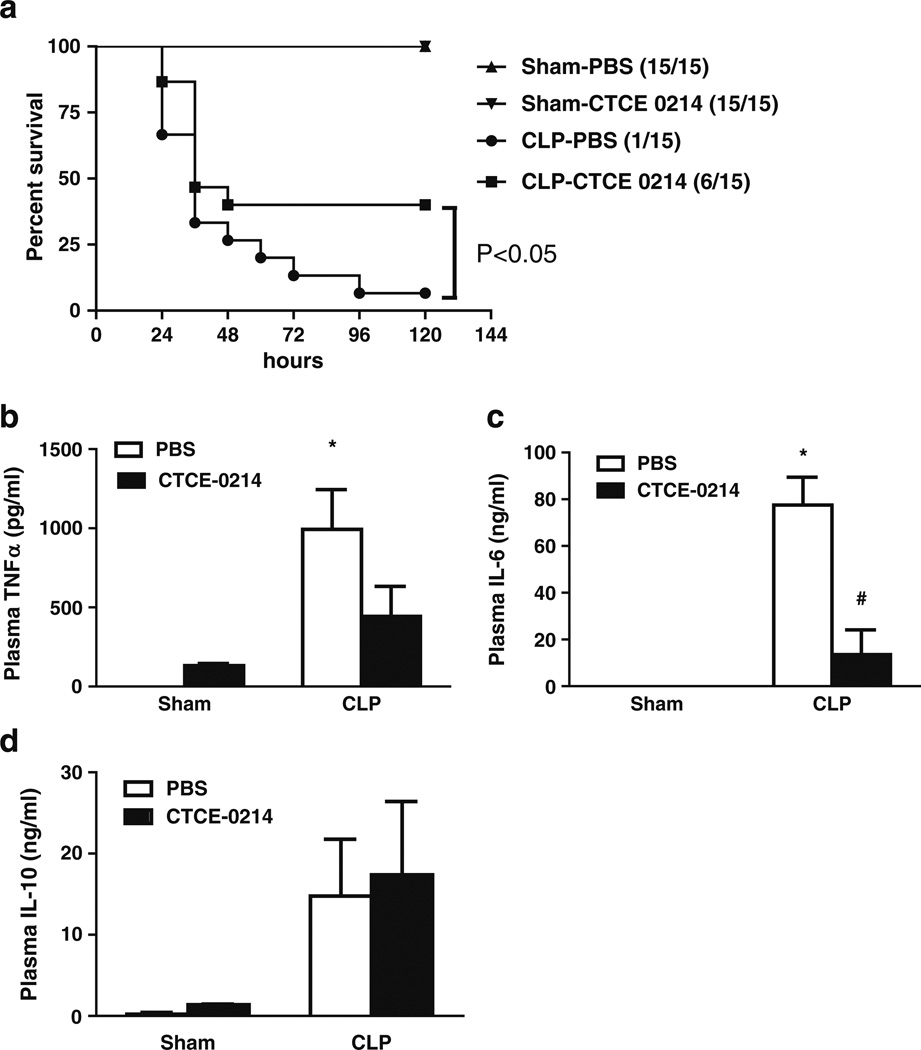
Fig. 4.
Effect of CTCE-0214 on severe CLP-induced sequelae. CD-1 mice were subjected to CLP in the absence of antibiotics. CTCE-0214 (25 mg/kg) or vehicle PBS was administrated by injection subcutaneously at 2, 18, 26, 42 and 50 h after CLP. Mortality was monitored every 12 h until 120 h (a). N=15/group. Statistics were determined with the log-rank (Mantel–Cox) test using GraphPad Prism software. *p<0.05 compared with the PBS group. CTCE-0214 (10 mg/kg) or vehicle PBS was also administrated by injection subcutaneously at 2 and 6 h after CLP. Plasma TNF-α (b), IL-6 (c), and IL-10 (d) were measured 24-h post-CLP. *p<0.05 compared with control, #p<0.05 compared with the PBS group. N=3–5.