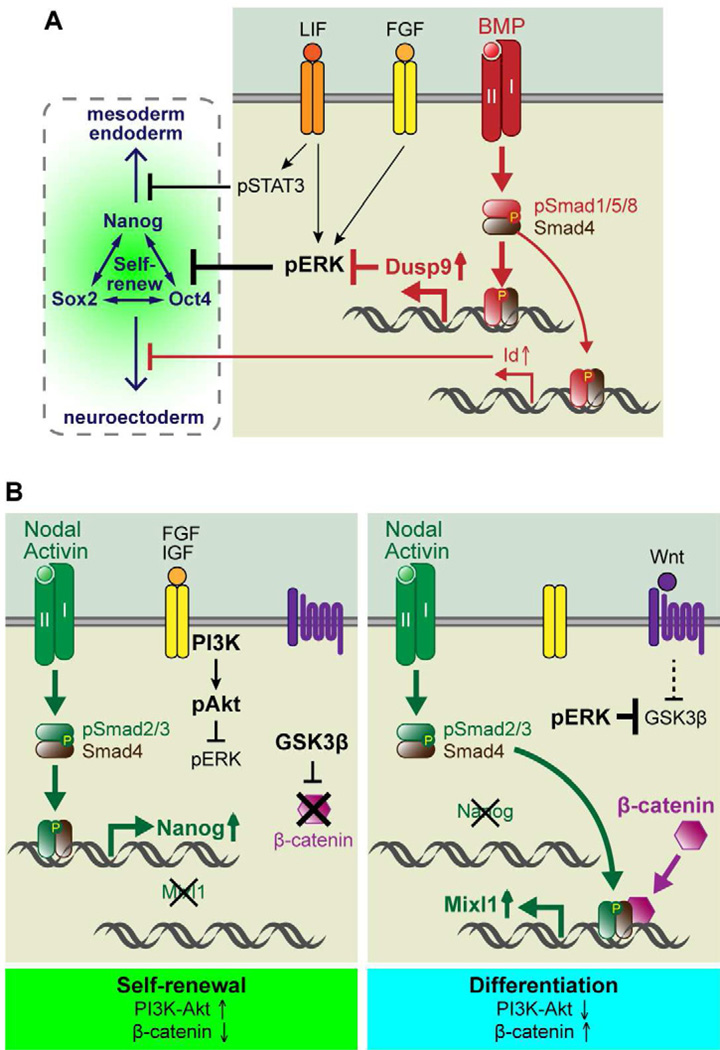
Figure 2. Signaling Crosstalk between TGF-β and Other Pathways in Mouse and Human ESCs.
(A) Self-renewal mechanisms in mouse ESCs. LIF and BMPs cooperate to maintain self-renewal. Tyrosine kinase receptor signaling, e.g. FGFs, typically activate downstream effectors ERK-MAPK, and induce differentiation. BMPs induce canonical Smad1/5/8 signaling pathways and one of their key targets for ESCs was recently shown to be the gene encoding ERK-specific phosphatase Dusp9. Dusp9 negatively affects ERK activity and hence support ESC self-renewal. Through the simultaneous induction of another BMP target gene, Id, and through activation of the STAT3 pathway by LIF, these two factors further promote ESC self-renewal by inhibiting mesendoderm and neuroectoderm differentiation, respectively.
(B) Self-renewal mechanisms in human ESCs. Nodal/Activin and hyperactive PI3K/Akt signaling cooperate to maintain self-renewal. Nodal/Activin induce canonical Smad2/3 signaling pathways and a key target for ESCs is Nanog. Hyperactive PI3K/Akt signaling can be achieved by some growth factors, such as FGF and IGFs, and this leads to suppression of ERK activity. When PI3K/Akt signaling diminishes, ERK inhibition is relieved, which in turn, suppresses the GSK3β kinase. This results in stabilization of β-catenin, which apparently associates with pSmad2/3-Smad4 to influence the target genes that become transcribed, thereby switching the ESC from a self-renewing to differentiating status.