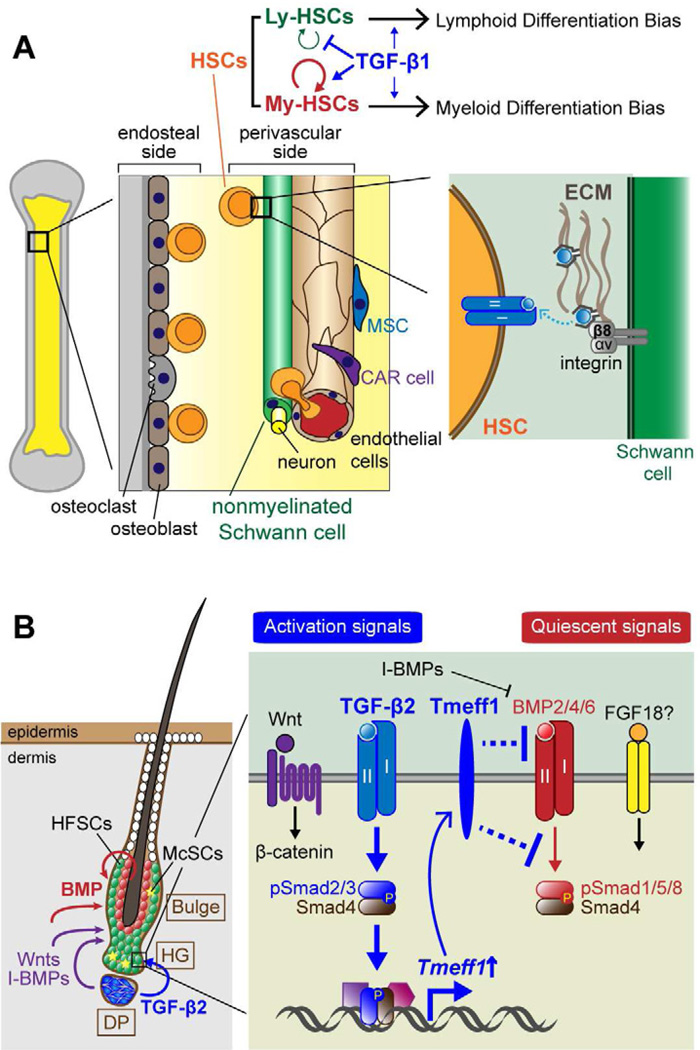
Figure 4. Diverse functions of TGF-β signaling in the control of adult stem cell behaviors.
(A) The bone marrow niche consists of a variety of cell types, which include hematopoietic stem cells (HSCs), mesenchymal stem cells, osteoblasts, endothelial cells, CXCL12-abundant reticular (CAR) cells, sympathetic neurons and nonmyelinating Schwann cells. HSCs located on the endosteal side of the niche tend to be quiescent, a feature necessary for their self-renewal and maintenance of stemness, while HSCs residing on the perivascular side are more active, priming them for differentiation. HSC quiescence is dependent upon active TGF-β, which appears to be released from its associated LAP through a mechanism involving β8-integrin, expressed on the surface of the nonmyelinated Schwann cells. TGF-β signaling also exacerbates the functional differences between the myeloid and lymphoid HSC subtypes. TGF-β enhances My-HSC self-renewal at low levels and drives myeloid differentiation at higher levels. By contrast, even at low levels, TGF-β induces Ly-HSC differentiation and inhibits their self-renewal.
(B) In the hair follicle, epithelial hair follicle stem cells (HFSCs) and melanocyte stem cells (McSCs) are located in a niche referred to as the bulge and hair germ (HG). Demarcating the dermis and epithelium is a basement membrane, and although the dermis contributes to niche signaling, HFSCs are restricted to the follicle side of the basement membrane. Sandwiched between the HFSC layer and the club hair is a layer of inner bulge cells (red circles) that emit high levels of BMP6 and FGF18 which maintain HFSC quiescence. Another key niche component is a transient one, the dermal papilla (DP), which only sits at the base of the bulge during the resting phase of the hair cycle, when HFSCs are in their most quiescent state. During this phase, crosstalk between HFSCs and DP change their transcriptional states and ultimately lead to TGF-β2 production by the DP, and pSmad2/3-Smad4 signaling by the adjacent HFSCs. A key downstream TGF-β2 signaling target is the gene encoding Tmeff1, which inhibits BMP signaling and lowers the threshold necessary to initiate HFSC self-renewal and fate specification necessary to launch a new round of hair growth.